Yoshiya INOKUCHI (Kyusyu Univ. and IMS), Yukito NAITOH, Kazuhiko OHASHI (Kyusyu Univ. and IMS), Ken-ichi SAITOW (Grad. Univ. for Advanced Studies), Keitaro YOSHIHARA and Nobuyuki NISHI (Kyusyu Univ. and IMS)
[Chem. Phys. Lett. 269, 298 (1997)]
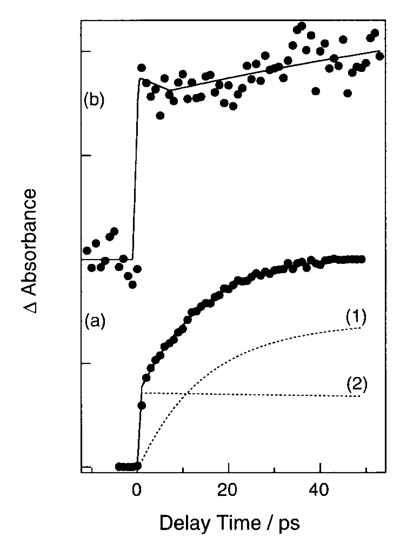
We have investigated a formation process of benzene dimer cation in neat liquid benzene by using a femtosecond pump-probe laser system. Third harmonic of a regeneratively amplified Ti:Sapphire laser was focused on a flow jet of neat benzene with a thickness of 100 (mu)m at room temperature. The absorption changes from visible to near IR are measured. Figure 1 shows the transient absorption signals at (a) 580 nm and (b) 920 nm. The rise curve at 580 nm is analyzed as two composites of (1) a slowly rising component with a time constant of 14 ps, and (2) a component with a fast rise within the time resolution of apparatus and a slow decay. Based on the pump power dependence of the signal intensity and transient absorption spectrum, we attribute the component (1) to benzene excimer and (2) to the S1 state of monomer benzene produced by one-photon absorption. The transients observed at 920 nm having a fast rise (< 1 ps) is assigned to benzene dimer cation formed by two-photon absorption. The most reasonable configuration of neat liquid benzene is perpendicular just between the nearest neighbors. The benzene excimer and the dimer cation, however, have a parallel structure. Therefore, the formation times include reorientation from a perpendicular to a parallel configuration. The difference in the appearance times can be rationalized by an effect of a positive charge created in the liquid. Since (pi) electrons of a neighboring benzene feel a strong attractive force from the positive charge, the reorientation to the parallel structure must occur much faster than the rotational motion of neutral species. This causes the fast formation of benzene dimer cation.

Figure 1. Transient absorption signals measured at (a) 580 nm and (b) 920 nm.