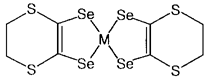
M(ddds)2
Hideki FUJIWARA, Emiko ARAI and Hayao KOBAYASHI
[J. Chem. Soc., Chem. Commun. 837 (1997)]
The selenium substitution of inner sulfur atoms of M(dddt)2 is expected to increase the transverse intermolecular interaction and therefore to stabilize metallic state. From this viewpoint, we synthesized novel metal complex [M(ddds)2]n- (M=Ni, Au; n=0, 1). The crystal structures of tetraalkylammonium salts of M(ddds)2 were determined. There is no stack of M(ddds)2 anions. The anions and the cations are alternately arranged. The intraligand Se-Se length is longer than that of inner sulfur atoms of M(dddt)2- and is almost equal to the S-S length in the six-membered ring. Therefore, two-dimensional intermolecular close contacts can be expected in M(ddds)2 complexes. Neutral M(ddds)2 (M = Ni, Au) were synthesized by chemical and electrochemical oxidation from monoanionic M(ddds)2. The neutral molecules pack as parallel crosses. Ni(ddds)2 dimer has intradimer Ni-Se bond (2.496(1)Å) and forms a [Ni(ddds)2]2 molecule. The Bu4N[Ni(ddds)2] shows two pairs of reversible redox waves (-0.60, +0.16 V vs. Ag/AgCl), which correspond to the two redox processes through its dianion to neutral state, and one irreversible redox wave. The room temperature magnetic susceptibility measurement showed that Bu4N[Ni(ddds)2] is paramagnetic with (mu)eff = 1.77 mB corresponding to one unpaired electron per molecular formula.

M(ddds)2