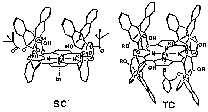
Formation of hydrogen bonding between a oxygen atom and proximal polar group is important in the stabilization of heme-O2 complexes. We prepared the Fe complex of 'single coronet' porphyrin (SC) bearing phenolic OH groups inside the cavity as a new heme protein model (Figure 1). This complex showed almost irreversible binding of O2 [P1/2(O2) < 10-5 Torr], which was the lowest value ever recorded. The O2 complexes stabilized by nearby OH groups. On the other hand, the corresponding CO complex was rather unstable [P1/2(CO) = 0.054 Torr]. from flash photolysis, rate constants of formation and dissociation of the CO complex were determined to be kB+CO = 2.5 ¥ 106 M-1 s-1 and kB-CO = 1.31 s-1. Thus, the CO complex of this model was destabilized than Mb and the related model complexes. The FT-IR and resonance Raman spectra also support this destabilization (Figure 2). The stretching frequencies of C-O and Fe-C were appeared at lower frequency region and they are explained by polar interaction between the OH groups and the binding CO.

Figure 1. Structure of the Fe derivatives of 'single-coronet' porphyrin (SC) and 'twin-coronet' porphyrin bearing thiolate ligand (TC). Double bonds are omitted for clarity.
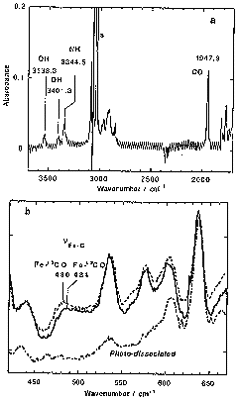
Figure 2. FT-IR (a) and resonance Raman spectra (b) of the SC-FeCO.
Cytochrome P-450 has an array of polar peptide residues around its O2-binding/activation site. These groups are assumed to play a key role in proton relay and the acceleration of O-O bond cleavage. As a model compound, we prepared the Fe complex of 'twin-coronet' porphyrin (TC) which have hydroxyl groups at the proximal position of the Fe ion inside one cavity and a thiolate ligand in other cavity (Figure 1). The CO complex showed characteristic visible absorption spectra lmax = 447 nm. Spectroscopic characterization of the corresponding O2 complex and its application to catalytic oxidation is in the way.
In cytochrome c oxidase (CcO), the CuB-heme a3 center performs four-electron reduction of O2 and promotes the resulting proton flow through membrane. Tris(pyridylmethyl)amino group (TPA)-linked porphyrin derivatives were prepared as its active-center model compounds. At lower temperature (-20°C). it formed the corresponding intermediary O2 complex (lmax = 420 nm), which was confirmed by ESI-MS [m/z = 1094 (16O2), 1098 (18O2)]. Upon warming this complex to 0°C, it changed to a new complex (lmax = 441 nm), which showed oxygen-active resonance Raman spectra at n = 822 cm-1 (D(16O2/18O2) = 39 cm-1). This band is not nO-O from the spectra with 16O18O. It is most likely FeIII-O-CuII or FeIV(=O).
Manganese porphyrin dimers having appropriate Mn-Mn separation showed catalytic activity toward O2 evolution by water oxidation under anodic oxidation conditions. In order to determine the active intermediate and the reaction mechanism of this reaction, oxidation of the dimanganese complex with peracid oxidation with mCPBA. Detailed analysis of the course of the oxidation revealed the stepwise formation of MnV(=O) intermediate through the corresponding manganese(III) perbenzoate complex. The compound is easily transferred to MnIV(OR)2 dimer in the presence of water or alcohol, which was assigned by ESI-MS, ESR, and elemental analysis. Thus, the transiently formed MnV(=O) rapidly transferred to the MnIV species, which is a good precursor to the corresponding MnV(=O) dimer.
back to Joint Studies Programs