By virtue of the progress in the molecular design of the two-dimensional p metals in 1980s, it becomes easy to prepare the organic conducting systems which retain metallic state down to low temperatures. In order to cultivate new field in the organic metal systems, we are now trying to obtain the electrically and magnetically active molecular systems. The simplest way to vest the magnetic properties to organic conductors is to use magnetic ions such as FeCl4- and CuCl42- as counter anions. In these systems, the highest occupied molecular orbital of organic molecule and d orbitals of anion are the incompletely occupied. Needless to say, the pi electrons convey the electrical current and the 3d electrons are mainly responsible for the magnetic properties.
We have recently examined several molecular magnets based on organic charge transfer complexes. Organic charge transfer complexes form a large variety of electric structures, which are governed by the transfer integrals and the on-site Coulomb interaction. The localized magnetic moments of d electrons embedded in insulating complexes are expected to interact with each other through superexchange interaction mediated by the organic p electron system, while localized d spins in metallic complexes are coupled to each other via pi-d interaction.
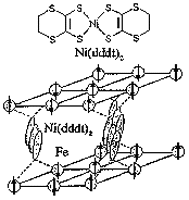
[Ni(dddt)2]3(FeBr4)2 is a typical semiconducting molecular magnets exhibiting the antiferromagnetic transition at 6 K. The susceptibility behavior indicates the three-dimensional nature of the spin system. Therefore the superexchange interlayer interaction through Fe-Br···[Ni(dddt)2]···Br-Fe should play an essential role in this system (Figure 1).

Figure 1. Schematic drawing of the magnetic structure of [Ni(dddt)2]3(FeBr4)2.
Owing to the unique redox nature of the chloranilate (CA), the one-dimensional transition metal complexes with CA as the bridging ligands (M-CA-M-) are expected to be interesting magnetic materials. We have recently synthesized linear complex with paramagnetic Cu2+ ions, [Cu(CA)(pyz)]n (pyz=pyrazine). The magnetic susceptibility indicated the weak antiferromagnetic interaction between Cu2+ ions (J=-3 cm-1). [M(CA)(H2O)2](phz)n (M=Mn, Fe: phz=phenazine) with high-spin metal atoms [S=5/2 (Mn), S=2 (Fe)] were also prepared. Considering the fairly high energy level of d orbitals of these metal atoms, it might be possible that the (CA3-)· radical could be produced by the electrochemical oxidation. Then the system will become one-dimensional ferrimagnet with strong Fe(S=5/2)···CA· (S=1/2) antiferromagnetic interaction.
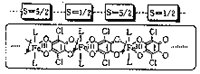
Figure 1. Schematic drawing of ferrimagnetic chain of [M(CA)(H2O)2](phz)n system.
back to Joint Studies Programs