Yoshiya INOKUCHI (Kyusyu Univ.), Yukito NAITOH, Kazuhiko OHASHI (Kyusyu Univ. and IMS), Ken-ichi SAITOW (Graduate Univ. for Advanced Studies), Keitaro YOSHIHARA, and Nobuyuki NISHI (Kyusyu Univ. and IMS)
[Chem. Phys. Lett. 269, 298 (1997)]
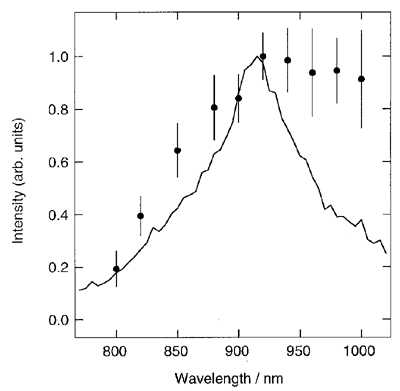
Photoionization of neat liquid benzene has been studied by femtosecond pump-probe experiments in the near infrared (IR) region. Third harmonic of a regeneratively amplified Ti:Sapphire laser was focused on a flow jet of neat liquid benzene with a thickness of 100 mm at room temperature. The absorption changes were measured in the region from visible to near IR. Figure 1 shows the transient absorption spectrum at 2 ps delay time. A transient species is formed within 1 ps following two-photon excitation of liquid benzene at 268 nm, which shows a broad absorption band with a peak at 920 nm and extended intensities at wavelengths around 1000 nm. Since the band position of the transient absorption spectrum nicely coincides with that of the photodissociation spectrum of benzene dimer cation in the gas phase, this band is attributed to a charge resonance band of a localized dimer cation created in neat liquid benzene. The asymmetric band shape may suggest that appreciable amount of the dimer cores are dressed in various sizes of benzene molecules.

Figure 1. Transient absorption spectrum of neat liquid benzene in a near infrared region (solid circles). Solid curve shows photodissociation spectrum of mass-selected benzene dimer cation in the gas phase.