Tatsuya TSUKUDA (Univ. Tokyo), Mark A. JOHNSON (Yale Univ.) and Takashi NAGATA
[Chem. Phys. Lett. 268, 429 (1997)]
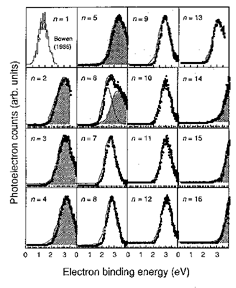
Since the pioneering work of Klots and Compton1), a number of experiments have shown that cluster anions of carbon dioxide, (CO2)n-, with n >= 3 are readily formed in the collisions between neutral (CO2)N clusters and slow electrons, while a bare CO2 molecule does not bind an excess electron as long as it retains the linear equilibrium geometry. We have re-investigated photoelectron spectra2) of (CO2)n- at the photon energy of 4.66 eV (Figure 1), from which the vertical detachment energy (VDE) is determined as a function of cluster size 2 =< n =< 16. The n-dependence of VDE exhibits sharp discontinuities between n = 6 and 7, and between n = 13 and 14. This indicates that the anionic core of (CO2)n- changes from CO2- to C2O4- at n = 7, and from C2O4- to CO2- at n = 14. A hole-burning type of photoelectron measurement has also been performed revealing that (CO2)6- is flopping between the two isomeric structures, CO2- ···(CO2)5 and C2O4- ···(CO2)4.

Figure 1. Photoelectron spectra of (CO2)n- with 2=< n =< 16. The photoelectron counts are plotted against electron binding energy defined as Eb = h(nu) Ek, where h(nu) and Ek represent the photon energy and the kinetic energy of the photoelectrons, respectively. The shaded areas correspond to the band components attributed to the C2O4- ··· (CO2)n-2 isomers.