Scheme 1.
Tatsuya TSUKUDA (Univ. Tokyo), Morihisa SAEKI (Univ. Tokyo and IMS) and Takashi NAGATA
[J. Phys. Chem. 101, 5103 (1997)]
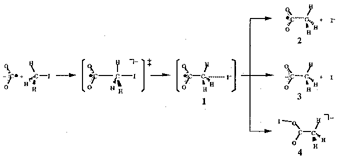
Since early 1970s, gas-phase SN2 displacement reactions, X + RY -> RX + Y, have long been a subject of extensive theoretical and experimental investigations. However, little is known about the reactions of larger analogues; that is, nucleophilic reactions including cluster anions, Mn + RY -> RMm + Y, etc. In the present study, we have demonstrated for the first time the chemical reactivity of (CO2)N as a nucleophile by means of a mass spectrometric technique combined with photoelectron spectroscopy. Negatively-charged clusters of carbon dioxide (CO2)N react with CH3I leading to the formation of anions with the formulae [(CO2)nCH3I], [(CO2)nCH3I] and [(CO2)nI]. Photoelectron spectroscopy of the product anions has revealed that an acetyloxy iodide anion, CH3CO2I, is formed in [(CO2)nCH3I] with 1 =< n =< 3. The CH3CO2I anion exhibits a large vertical detachment energy (VDE) of 3.53 eV, due probably to delocalization of the excess electron. Ab initio calculations show that the excess electron resides in the O-I anti-bonding orbital of CH3CO2I (see V-L-7). The reaction is found to be strongly subject to steric hindrance around the carbon site of CH3I. In fact, the reaction of (CO2)N with 2-C3H7I yields no product cluster anions containing 2-C3H7CO2I. We infer from this finding that CH3CO2I is formed via an SN2 transition state, which is prepared by a nucleophilic attack of CO2 on the carbon site of alkyl iodide (Scheme 1).
The overall reaction process can be regarded as carboxylation by the reductive activation of CO2, which opens up a possibility of studying elementary processes of electrochemical reactions, not in the condensed phase but in the gas phase with a restricted number of solvent molecules.

Scheme 1.