Scheme 1
Akiko INAGAKI (Tokyo Inst. Tech), Yoshiaki TAKAYA (Tokyo Inst. Tech), Toshifumi TAKEMORI (Tokyo Inst. Tech), Hiroharu SUZUKI (Tokyo Inst. Tech), Masako TANAKA (Tokyo Inst. Tech) and Masa-aki HAGA
[J. Am. Chem. Soc. 119, 625 (1997)]
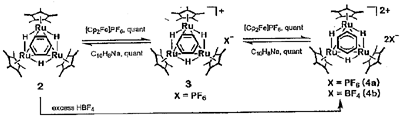
Transition metal complexes with facial arene ligands have been of great interest since these compounds may be reasonable models of arenes chemisorbed at a three-fold site on the surface of a close-packed metal lattice. We recently synthesized a novel facial benzene complex {(C5Me5)Ru}3((mu)-H)3((mu)3-(eta)2:(eta)2:(eta)2-C6H6) (1)by the reaction of {(C5Me5)Ru}3((mu)-H)3((mu)3-H)2 with 1,3-cyclohexadiene during studies of substrate activation on di- and trinuclear polyhydride complexes of ruthenium. The (mu)3- (eta)2:(eta)2:(eta)2-coordination of the benzene ring was confirmed by X-ray diffraction. Average "coordinated" and "uncoordinated" C-C distances of C6H6 ligand are almost equal within the experimental error. In the cyclic voltammogram recorded in tetrahydrofuran, two reversible one-electron oxidation were observed at -490 and -224 mV vs Ag/AgCl. In view of the interest in the correlation between oxidation state and structure of the face-capping benzene complex, oxidation with 1 and 2 equiv of ferricinium salt gave monocation and dication, respectively, in which the structure of dication was determined by X-ray crystallography of a single crystal of {(C5Me5)Ru}3((mu)-H)3((mu)3-(eta)3:(eta)3-C6H6)(BPh4)2(2)(Scheme 1). The most striking structural feature is the (mu)3-(eta)3:(eta)3-coordination of the C6 ring. An allyoiety is coordinated to one of three ruthenium centers, and another allyl moiety bridges two ruthenium centers. Therefore, complex 2 is the first example of the hapticity change in face-capping benzene ligand induced by a redox process. This dication 2 is reduced stepwise to form complex 1 quantitatively via the monocation by treatment with sodium naphthalide.

Scheme 1