Kazushi MASHIMA (Osaka Univ.), Naoki KOMURA (Osaka Univ.), Tsuneaki YAMAGATA (Osaka Univ.), Kazuhide TANI (Osaka Univ.) and Masa-aki HAGA
[Inorg. Chem. 36, 2908 (1997)]
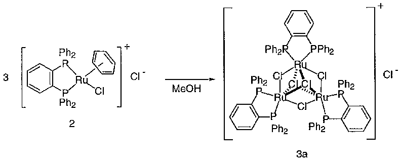
Treatment of [RuCl2((eta)6-C6H6)]2 with two equiv of 1,2-bis(diphenylphosphino)benzene (dpb) in methanol at room temperature afforded a cationic mononuclear complex [RuCl((eta)6-C6H6)(dpb)]Cl (2). When complex 2 in methanol was heated at 50 °C for 13 h, a cationic trinuclear complex [Ru3((mu)2-Cl)3((mu)3-Cl)2(dpb)3]Cl (3a) was obtained in 75% yield with a liberation of the benzene ligand. The cationic trinuclear core was revealed by X-ray analysis of [Ru3((mu)2-Cl)3((mu)3-Cl)2(dpb)3]PF6 (3b). The triangle of three ruthenium atoms were linked by three (mu)2-bridging chloride atoms and two face-capped (mu)3-chloride atoms. Each ruthenium was surrounded with four chloride atoms and two phosphorus atoms in cis positions and thus adopted pseudo octahedral geometry as expected for Ru(II) complexes. The interatomic Ru-Ru distances (3.219(3)-3.234(3) Å) of 3b are out of the Ru-Ru single bond distance.
Cyclic voltammetry of 3b in THF (0.1 M TBABF4) showed the reversible one-electron oxidation process at + 0.75 V vs Fc+/Fc. In addition, the irreversible reduction occurred at - 1.92 V. The oxidation potential of complex 3b is almost comparable to face-sharing bioctahedral Ru complexes such as [Ru2Cl3(PEt2Ph)6]+ (+0.78 and +1.33 V vs Fc+/Fc). In the case of face-sharing bioctahedral Ru complexes, two oxidation processes were observed with the potential separation of ~ 0.5 V, indicating the strong interaction of Ru-Ru ions through strong delocalization of the charge. In the present trinuclear complex, attempts to measure the additional oxidation waves in more positive potential region were unsuccessful because of the experimental limit for available potential window. However, the substantial charge delocalization will be expected in 3b. A wide gap between oxidation and reduction indicates the strong interaction between metal ions and the stabilization of oxidation state of all Ru(II) ions in complex 3b, which is attributed to the coordination of the chelating triaryl phosphine ligand.
