Md. D. HOSSAIN, Masa-aki HAGA, Hideaki MONJUSHIRO, B. GHOLAMKHASS (Osaka Univ.), Koichi NOZAKI (Osaka Univ.) and Takeshi OHNO (Osaka Univ.)
[Chem. Lett. 573 (1997)]
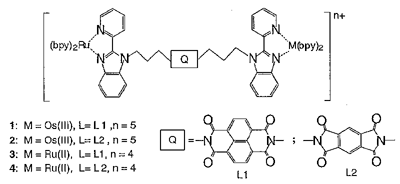
The photoinduced electron transfer processes in novel Ru(II)(bpy)2/Os(III)(bpy)2-based triad complexes containing functionalized diimide ligands in Scheme 1 were investigated by the picosecond time-resolved absorption(TA) spectra. The picosecond time-resolved absorption(TA) spectra of homodinuclear Ru(II)-L1-Ru(II) complex 3 in CH3CN observed at 15ps after excitation exhibits the bleaching of an MLCT band at 459 nm and the rise of absorption around 400 and 550 nm due to the anion radical of bpy ligand, indicating the formation of an MLCT triplet excited state of the Ru(II) moiety. In 200 ps, several absorption peaks appeared at 470, 610, 682, and 756 nm, which are characteristics of the L1 anion radical. These new TA peaks then disappeared with a single exponential decay (rate constant = 2.9 x 109 s-1) through back electron transfer to reproduce the original form. The yield of formation of the CS state (Ru(III)-(L1-)-Ru(II)) is calculated as 80-100% and the rate constants of the photoinduced electron transfer between the Ru(bpy)2 and L1 for complex 3 are determined as (1.1 ± 0.1) x 1010 s-1 and 2.9 x 109 s-1 from the excited state decay and the charge separation yield. The Ru(II)-L1-Os(III) mixed-valent complex 1 was selectively formed by one-electron oxidative electrolysis of Ru(II)-L1-Os(II) complex (3 x 10-4 mol dm-3) at +0.5V in CH3CN. The TA spectrum of complex 1 immediately after the excitation shows the formation of Ru(II)-MLCT excited state as shown in Figure 1. The fast electron transfer from L1- anion to Os(III) site gave a CS Ru(III)-L1-Os(II) state, which is the redox isomer state of the starting complex. The back electron transfer from L1- anion to Ru(III) site would reduce the production yield of the CS state that was estimated 75%. By considering the similarity of TA spectra for the early stage in both homodinuclear Ru complex 3 and mixed-valence complex 1, the electron transfer occurred from Ru(II) to L1 at first, followed by rapid electron transfer from L1 anion radical to Os(III). The rate of charge recombination(CR) from Os(II) to Ru(III) is calculated as 9.1 x 106 s-1 ((tau) =110 ns). This slow CR rate implies the diimide unit keeps the Ru(III) and Os(II) moieties apart from each other even though three donor or acceptor units are connected by a somewhat flexible propane linker. The direct photoinduced electron transfer process from Ru(II)-MLCT excited state to Os(III) is much slower compared to the stepwise electron transfer.

Scheme 1. Newly synthesized Ru(II)(bpy)2/Os(III)(bpy)2-based triad complexes containing functionalized diimide ligands
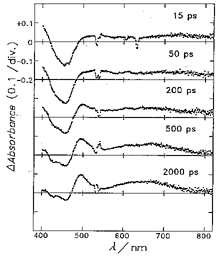
Figure 1. Picosecond time-resolved transient absorption spectra of complex 1, [Ru(bpy)2(L1)Os(bpy)2](ClO4)5, in CH3CN (3 x 10-4 mol dm-3) at room temperature.