Hiroshi NAKAJIMA, Kiyotsuna TOYOHARA, Kiyoshi TSUGE and Koji TANAKA
[Chem. Lett. 485 (1996)]
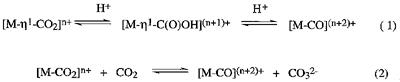
Much attention is being devoted to metal-(eta)1-CO2 complexes which are the key intermediate for CO2/CO conversion. In protic media, CO2/CO conversion on metals takes place through acid-base equilibria among [M-(eta)1-CO2]n+, [M-(eta)1-C(O)OH](n+1)+ and [M-CO](n+2)+ (eq 1). In the absence of proton donors, CO2/CO conversion is achieved by oxide transfer between metal-CO2 and CO2 (eq 2), which may be more

useful than that in H2O (eq 1) from the viepoint of utilizartion of CO2 as C1 resources. Reversible oxide transfer reactions from CO32- to [Ru(bpy)2(CO)2]2+ and from Ru(bpy)2(CO)((eta)1-CO2) to CO2 are established (Scheme 1). The oxide transfer reaction from CO32- to [Ru(bpy)2(CO)2]2+ producing Ru(bpy)2(CO)((eta)1-CO2) and CO2 in CH3CN is ascribed to the less basicity of the Ru-(eta)1-CO2 group compared with that of CO32-. The reverse oxide transfer from Ru(bpy)2(CO)((eta)1-CO2) to CO2 affording [Ru(bpy)2(CO)2]2+ is assisted by Li+ due to low solubility of Li2CO3 in CH3CN.