Jie DAI,a Mikiko YAMAMOTO,a Takayoshi KURODA-SOWA,a Masahiko MAEKAWA,a Yusaku SUENAGAa and Megumu MUNAKATA (aKinki Univ. and IMS)
[Inorg. Chem. 36, 2688 (1997)]
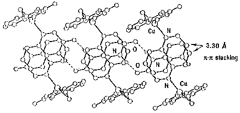
Copper(I) ethylene complex of 2-hydroxyquinoxaline (Hhq), [Cu(Hhq)2(C2H4)]ClO4, was synthesized and characterized crystallographically. The cations of the complex are linked to each other by head-to-tail double H-bonded, AD:::DA, to form a one-dimensional infinite zig-zag chain structure. Keto form (C=O...H-N) is predominant in these H-bonds and the N-O distance is 2.79 Å. The H-bond assembled Hhq molecule planes are strongly stacked with the distance of 3.30 Å forming a unique two dimensional cooperative system of H-bonds and (pi)-(pi) interactions (Figure 1), which is a fundamental characteristic of proton-electron transition systems. The NMR and IR spectra of this complex are also discussed.

Figure 1. The packing of the coordinarion and H-bond-linked chains in [Cu(Hhq)2(C2H4)]ClO4.