Mikiko YAMAMOTO,a Xinmin GAN,a Takayoshi KURODA-SOWA,a Masahiko MAEKAWA,a Yusaku SUENAGAa and Megumu MUNAKATA (aKinki Univ. and IMS)
[Inorg. Chim. Acta 261, 169 (1997)]
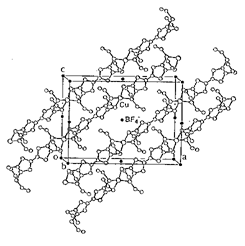
Linear copper(I) coordination polymers, [Cu-(TTC2-TTF)]BF4 (1) and [Cu(TTC3-TTF)]ClO4 (2), were synthesized and characterized by spectroscopic and X-ray crystallographic methods. They are 1:1 metal-ligand complexes in which each of the copper(I) ions is tetrahedrally coordinated by four sulfur atoms from the bridging TTC2-TTF or TTC3-TTF molecules to form coordination polymeric chains, Figure 1. 1 and 2 were doped with iodine to afford {[Cu(TTC2-TTF)]BF4}(I3)0.33 and {[Cu(TTC3-TTF)]ClO4}(I3)0.33, which exhibit conductivities of 2 x 10-5 and 4 x 10-5 Scm-1 at 25 °C for compacted pellets, respectively.

Figure 1. The structure of [Cu(TTC2-TTF)]BF4 (1).