Liang Ping WU,a Yoshiro YAMAGIWA,a Takayoshi KURODA-SOWA,a Tadao KAMIKAWAa and Megumu MUNAKATA (aKinki Univ. and IMS)
[Inorg. Chim. Acta 256, 155 (1997)]
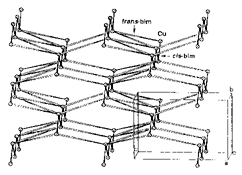
The synthesis and characterization of the copper(II) complex [Cu(bim)2.5](ClO4)2·2MeOH (bim = 1,2-bis(imidazol-1'-yl)ethane) is described. The complex contains non-interacting ClO4- anions and {[Cu(bim)2.5]2+}n macrocation in which each metal ion involves a distorted square-based pyramidal coordination environment comprising five N atoms from five separate bim molecules. The total structure consists of two alternately stacked 2-D sheets of copper(II) ions bridged through the bidentate imidazole molecules constituting a three-dimensional network (Figure 1).

Scheme 1. Perspective view of the [Cu(bim)2.5]2+ cation. Circle, white and black rods represent the copper center, bim molecules of trans and cis-configurations, respectively.