Masaaki OKAZAKI (Tohoku Univ.), Hiromi TOBITA (Tohoku Univ. and IMS) and Hiroshi OGINO (Tohoku Univ.)
[Chem. Lett. 437 (1997)]
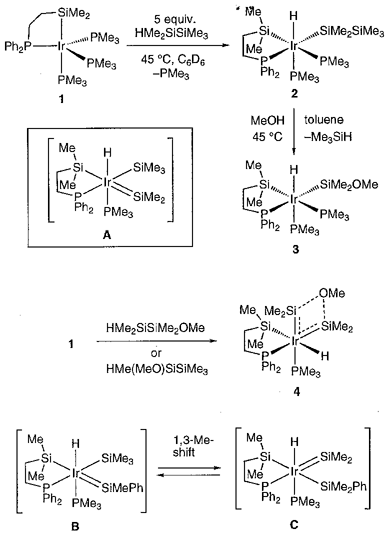
Reactions of an Ir(I) complex Ir{(eta)2-Me2Si(CH2)2PPh2}(PMe3)3 (1) with several hydrodisilanes have been investigated. Treatment of 1 with 1 equiv of HSiMe2SiMe3 at room temperature gave a hydrido(disilanyl)iridium(III) complex 2. Complex 2 was then cleanly converted to 3 on heating in MeOH at 45 °C. A plausible mechanism involves the formation of silyl(silylene) complex A followed by the addition of MeOH to the Si=Ir double bond. The formation of silyl(silylene) complex was confirmed by the reaction of 1 with HSiMe2SiMe2OMe at 45 °C. By this reaction, the silyl(silylene) intermediate was stabilized by the coordination of an internal donor, and bis(silylene) complex 4 was formed cleanly. Complex 4 is the first isolated bis(silylene) complex of a group 9 metal. Complex 1 can catalyze redistribution of substituents in hydrodisilanes. Thus, thermolysis of HMePhSiSiMe3 in the presence of a catalytic amount of 1 resulted in the isomerization to give a mixture of HMePhSiSiMe3 and HMe2SiSiMe2Ph in the ratio of 2 : 3. The converse isomerization was also observed by the reaction of 1 with HMe2SiSiMe2Ph. This isomerization reaction can be explained by the mechanism involving silyl(silylene) complexes B and C, on which the 1,3-Me-shift occurs efficiently. In the reaction of 1 with HMe(MeO)SiSiMe3, a similar 1,3-Me-shift occurs in the course of the reaction to give 4 cleanly.
