Hiroaki WADA (Tohoku Univ.), Hiromi TOBITA (Tohoku Univ. and IMS) and Hiroshi OGINO (Tohoku Univ.)
[Organometallics 16, 2200 (1997)]
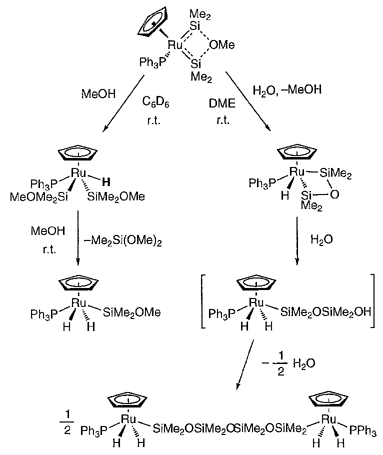
The reactivity of a donor-stabilized bis(silylene)ruthenium complex Cp(Ph3P)Ru{SiMe2···O(Me)···SiMe2} (1) toward methanol and water has been investigated. Reaction of 1 with MeOH afforded an addition product trans-Cp(Ph3P)RuH(SiMe2OMe)2 (2) in high yield. Complex 2 reacted further slowly with an excess of MeOH to give trans-Cp(Ph3P)Ru-(H)2(SiMe2OMe) (3) and Me2Si(OMe)2. The reaction of 1 with MeOH is considered to be initiated by nucleophilic attack of MeOH at the strongly electrophilic silylene ligand. Thus, the slower rate of the reaction of MeOH with 2 compared to that with 1 indicates that the electrophilicity of a silyl ligand in 2 is lower than that of a slilylene ligand in 1. In contrast, the reaction of 1 with H2O yielded first the metallacycle cis-Cp(Ph3P)Ru(H)((eta)2-SiMe2OSiMe2) (4), probably through the intramolecular condensation of SiMe2OH with SiMe2OMe. Complex 4 was then converted to the tetrasiloxane complex trans,trans-[Cp(Ph3P)Ru(H)2SiMe2OSiMe2]2O (5) by the action of an excess of H2O in DME. In a possible mechanism of the last reaction, the chelate ring of 4 is opened at the Ru-Si bond by the nucleophilic attack of H2O to form Cp(Ph3P)Ru(H)2SiMe2OSiMe2OH, which then undergoes condensation with dehydration to give 5. These results indicate that the silylene ligands of 1 is extremely electrophilic and readily attacked even by a weak nucleophile such as methanol or water.
