Yoshiro YAMASHITA, Masaaki TOMURA and Kenichi IMAEDA
[Chem. Commun. 2021 (1996)]
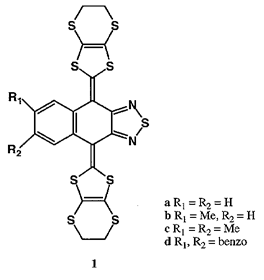
Organic conductors are usually obtained from planar molecules showing multi-redox properties. For the non-planar molecules, ion radical states are in general unstable due to the limited conjugation, and two-electron redox is often observed. We recently prepared non-planar bis(1,3-dithiole) donor 1d which gave metallic cation radical salts. In an extension of that work, we have now found that donors 1a-c showing two-electron oxidation at one stage afforded cation radical salts incorporating solvent molecules. The cation radical salts of 1a-c were isolated as single crystals when they were electrochemically oxidized in the presence of Bu4NBF4 in tetrahydrofuran (THF). The composition of the salts of 1b and 1c was 2:1:1 (donor:PF6:THF), while 1a gave a salt with a ratio of 1:1:1. The salt of 1c showed a metallic temperature dependence of conductivity down to 180 K. Next the included THF molecule was replaced by 2,5-dihydrofuran (DHF) or 1,3-dioxolane (DO) to examine the effect of the included molecules. The room temperature conductivities of the DHF and DO included salts were the same as that of the THF one. However, they showed semiconducting behaviour with the different activation energies (0.19 eV for the DHF salt and 0.03 for the DO one). Therefore, the stability of the metallic state decreases in the included solvent order: THF < DO < DHF.
