Akira OHTA and Yoshiro YAMASHITA
[Mol. Cryst. Liq. Cryst. 296, 1 (1997)]
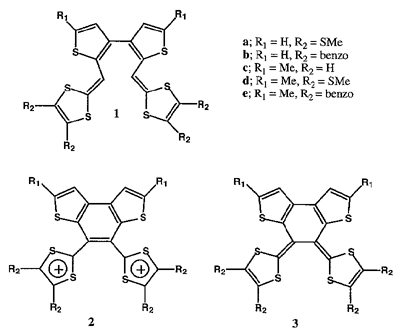
2,2'-Bis(1,4-dithiafulven-6-yl)-3,3'-bithienyls 1 were prepared by using either a Wittig or a Wittig-Horner reaction. The cyclic voltammograms of 1 revealed that they undergo an intramolecular cyclization by electrochemical oxidation. Chemical oxidation of 1 with tris(4-bromophenyl)aminium hexachloroantimonate gave intramolecular cyclization products 2 as dication salts. The hexachloroantimonate ion was found to act as an oxidizing agent in the reaction. Chemical reduction of the dication salts with zinc afforded the corresponding neutral bis(1,3-dithiole) donors 3 in high yields. The cyclic voltammograms of 3 showed reversible one-stage two-electron oxidation waves, and the potentials are fairly low. A conductive TCNQ complex was formed from one of them. X-ray analyses of the neutral and dication states of the donor show that there is a large conformational change between them. In contrast to the neutral molecule, in the dication the two 1,3-dithiole rings are almost orthogonal to the completely planar tricyclic system.
