Akira OHTA and Yoshiro YAMASHITA
[Heterocycles 44, 263 (1997)]
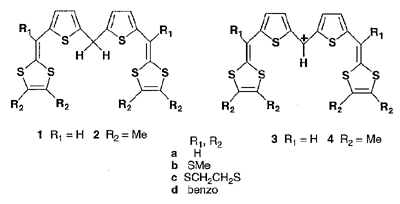
Novel bis(1,3-dithiole) compounds 1 and 2 containing a di(2-thienyl)methane unit have been prepared by either a Wittig or a Wittig-Horner reaction. These compounds showed irreversible cyclic voltammetric behavior. Oxidation products of 1 and 2 were strongly dependent on the substituents (R1) on the C6-carbon atoms of the 1,4-dithiafulvenyl groups. In the case of R1 = H (1), intermolecular coupling giving a redox-active oligomer took place. On the other hand, compounds 2 containing methyl substituents underwent deprotonation by oxidation to give the corresponding dithienylmethyl cations 4 which were also obtained by hydride abstruction of 2 with trityl tetrafluoroborate in dichloromethane. The cations show intense absorptions in the near-infrared region (> 900 nm in MeCN). The alkylthio groups on the 1,3-dithiole rings make the absorptions much red-shifted. The cation 3b was prepared by the hydride abstruction method. The single crystal was obtained by recrystallization from acetonitrile. X-ray structure analysis revealed that the thienyldithiafulvenyl skeleton is almost planar and the bond alternation is decreased, indicating the polymethine cyanine-like structure of the cation. The cations form a dimer which is connected by the counteranion BF4- with short F···H contacts to construct a ladder-like network.
