Chitoshi KITAMURA and Yoshiro YAMASHITA
[J. Chem. Soc. Perkin Trans. 1, 1443 (1997)]
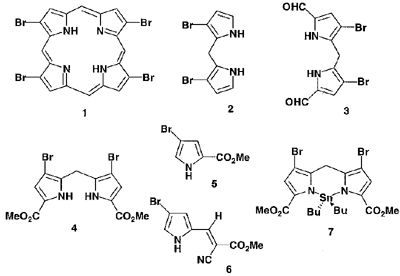
Most of the porphyrins prepared so far are meso-substituted compounds and b-substituted meso-non-substituted porphyrins have been rarely reported. We have now attempted to synthesize b-bromoporphyrin 1 using the reaction of dihydrodipyrrins 2 and 3. The dibromodihydrodipyrrin diester 4 was first prepared by reaction of the corresponding pyrrole 5 with dimethoxymethane and BF3·Et2O. Subsequently 4 was converted to 2 in moderate yield. The diformyldihydrodipyrrin 3 was readily prepared by methylation of the pyrrole 6 with BF3·Et2O, followed by deprotection. Attempted synthesis of the porphyrin 1 from 2 and 3 has been unsuccessful because of the low reactivity of 2. The reaction of 4 with hexabutylditin in the presence of a Pd catalyst produced a new type of tin complex 7 in low yield. This complex was also obtained by an alternative procedure in high yield and found to revert to 4 upon reaction with TFA.
