Yoshiro YAMASHITA, Katsuhiko ONO, Masaaki TOMURA and Shoji TANAKA
[Tetrahedron 53, 10169 (1997)]
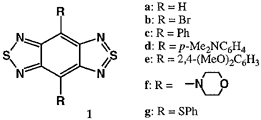
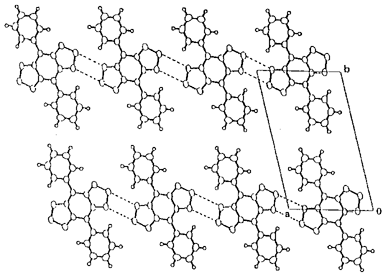
Benzo[1,2-c:4,5-c']bis([1,2,5]thiadiazole) (1a) containing a hypervalent sulfur atom has a low LUMO level. The several derivatives 1b-g were prepared here. The synthesis of the aryl derivatives were accomplished by using a Stille coupling reaction. The selenadiazole analogues were also synthesized. The electron accepting properties of these nonclassical heterocycles were shown by their high reduction potentials. The reduction potential of the dibromo derivative 1b is comparable to that of p-benzoquinone. Introduction of electron-donating groups into the electron-withdrawing heterocycle afforded novel donor-acceptor compounds. Their cyclic voltammograms show that they are easily both oxidized and reduced. The amino derivatives 1d and 1f have the absorption maxima above 700 nm due to the small HOMO-LUMO separation. X-ray structure analysis of the diphenyl derivative 1c revealed the formation of a tape-like network through short S···N contacts as shown in Figure 1.


Figure 1. Crystal structure of 1c, broken line S···N contacts (3.26Å).