Presenters
Naokazu Kano (Department of Chemistry, Univ. of Tokyo)
Takayuki Kawashima (Department of Chemistry, Univ. of Tokyo)
Shigeru Nagase (Department of Theoretical and Computational Molecular Science, Inst. for Molecular Science)
Abstract
In organic compounds, carbon–carbon bonds get progressively weaker as both the number of branches and the extent of steric congestion around the carbon–carbon bonds increase. In addition, introduction of the same multiple anionic or cationic charges usually results in electronic repulsion. Silicon is located below carbon in group 14 of the periodic table, and silicon–silicon bonds form the framework of many organosilicon compounds and the crystalline silicon of semiconductors. Although silicon can form pentacoordinated structure, no compounds containing a direct bond between two pentacoordinated silicon atoms of anionic halves have been reported. Such a bond is anticipated to dissociate because of the steric congestion around the silicon–silicon bond and the electronic repulsion between the anionic halves. We succeeded in synthesizing new dianionic species bearing a direct bond between two pentacoordinated silicon moieties. The species were found to be stable even under thermal conditions, contrary to the intuitive anticipation of silicon–silicon bond cleavage. Two pentacoordinated silicons, positively charged despite the formal negative charge, constitute a single bond and bind eight negatively charged atoms. Considering the structure and properties of the new silicon–silicon bond, we named it ‘Asura bond’ after Asura in Buddhism.
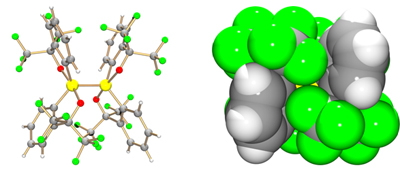
Figure caption. The crystal structure of the dianion moiety of the new pentacoordinated silicon–silicon bond species in the ball-and-stick view (left). Each yellow-colored silicon atom binds to two red-colored oxygen atoms, two grey-colored carbon atoms, and the other silicon atom. Yellow-green colored balls show fluorine atoms of the bidentate ligands. The space-filling view of the dianion moiety shows that the ligands surround two silicon atoms effectively (right).
Paper Information
Naokazu Kano, Hideaki Miyake, Keishi Sasaki, Takayuki Kawashima, Naomi Mizorogi and Shigeru Nagase, "Dianionic species with a bond consisting of two pentacoordinated silicon atoms", Nature Chemistry (DOI: 10.1038/NCHEM.513).

