
Professor Koichi Kato and Dr. Maho Yagi at Institute for Molecular Science and Okazaki Institute for Integrative Bioscience, National Institutes of Natural Sciences have conducted NMR analyses of the amyloid b (Aβ), an important component implicated in Alzheimer’s disease (AD), with ganglioside clusters and successfully provided a structural basis for this pathogenic interaction associated with AD.
Growing evidence has indicated that gangliosides interact with Aβ peptide on neuronal cell surfaces and thereby promote its assembly, which is considered as a crucial step in AD. The structural characterization of the GM1-interacting Aβ species is crucial for understanding the molecular mechanisms underlying the onset and the development of AD. Hence, NMR studies of the interactions between gangliosides and Aβ were conducted using the 920 MHz ultra-high field NMR spectrometer. The NMR data revealed that the sugar–lipid interface is primarily perturbed upon binding of Aβ, underscoring the importance of the inner part of the ganglioside clusters for accommodating Aβ. The NMR data obtained by using isotopically labeled Aβ bound to GM1 micelles also demonstrated that Aβ lies on hydrophobic/hydrophilic interface of the ganglioside cluster exhibiting an up-and-down topological mode in which the two a-helices and the C-terminal dipeptide segment are in contact with the hydrophobic interior, whereas the remaining regions are exposed to the aqueous environment. Furthermore, it was revealed that the thioflavin T-reactive b-structure is more populated in Aβ under conditions where the Aβ density on GM1 micelles is high. These findings indicate that the ganglioside clusters serve as a unique platform for binding coupled with conformational transition of Aβ molecules, rendering their spatial rearrangements restricted to promote specific intermolecular interactions leading to Aβ fibrillogenesis.
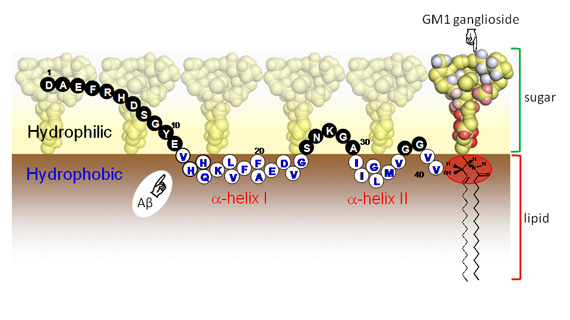 Schematic drawing of Aβ lying on the hydrophobic/hydrophilic interface of ganglioside clusters. The amino acid residues exposed to the hydrophilic and hydrophobic milieus are represented by closed and open circles, respectively, with single-letter codes. The sugar hydrogen atoms coloured in red and the ceramide hydrogen atoms in the area surrounded red ellipsoid exhibit attenuation of peak intensity in the presence of the C-terminally spin-labelled Aβ, indicating that they are in close spatial proximity of its C-terminus of Aβ.
Schematic drawing of Aβ lying on the hydrophobic/hydrophilic interface of ganglioside clusters. The amino acid residues exposed to the hydrophilic and hydrophobic milieus are represented by closed and open circles, respectively, with single-letter codes. The sugar hydrogen atoms coloured in red and the ceramide hydrogen atoms in the area surrounded red ellipsoid exhibit attenuation of peak intensity in the presence of the C-terminally spin-labelled Aβ, indicating that they are in close spatial proximity of its C-terminus of Aβ.
Paper Information
[1]Glycoconjugate Journal, 26, 999-1006 (2009)
Up-and-down topological mode of amyloid Aβ-peptide lying on hydrophilic/hydrophobic interface of ganglioside clusters
Maho Utsumi, Yoshiki Yamaguchi, Hiroaki Sasakawa, Naoki Yamamoto, Katsuhiko Yanagisawa and Koichi Kato
[2] FEBS Letters, 584, 831-836 (2010)
NMR characterization of the interaction between lyso-GM1 aqueous micelles and amyloid b
Maho Yagi-Utsumi, Tomoshi Kameda, Yoshiki Yamaguchi and Koichi Kato
[3]International Journal of Alzheimer’s Disease, ID 925073 (2011)
Spectroscopic characterization of intermolecular interaction of amyloid Aβ promoted on GM1 micelles
Maho Yagi-Utsumi, Koichi Matsuo, Katsuhiko Yanagisawa, Kunihiko Gekko and Koichi Kato

 Schematic drawing of Aβ lying on the hydrophobic/hydrophilic interface of ganglioside clusters. The amino acid residues exposed to the hydrophilic and hydrophobic milieus are represented by closed and open circles, respectively, with single-letter codes. The sugar hydrogen atoms coloured in red and the ceramide hydrogen atoms in the area surrounded red ellipsoid exhibit attenuation of peak intensity in the presence of the C-terminally spin-labelled Aβ, indicating that they are in close spatial proximity of its C-terminus of Aβ.
Schematic drawing of Aβ lying on the hydrophobic/hydrophilic interface of ganglioside clusters. The amino acid residues exposed to the hydrophilic and hydrophobic milieus are represented by closed and open circles, respectively, with single-letter codes. The sugar hydrogen atoms coloured in red and the ceramide hydrogen atoms in the area surrounded red ellipsoid exhibit attenuation of peak intensity in the presence of the C-terminally spin-labelled Aβ, indicating that they are in close spatial proximity of its C-terminus of Aβ.