
Assoc. Prof. Yuji Furutani at Institute for Molecular Science, Assoc. Prof. Takeshi Murata at Chiba University and Prof. Hideki Kandori at Nagoya Institute of Technology revealed the ion-protein interactions of a pharmaceutical target protein, V-ATPase, by using attenuated total reflection Fourier transform infrared (ATR-FTIR) spectroscopy.
Membrane proteins take a main role on homeostasis of living cells, which work as ion channel, ion pump, various types of chemical and biophysical sensors, and so on. Their molecular mechanisms have not been studied well, because X-ray crystallography and NMR spectroscopy are not easily feasible to them in general. ATR-FTIR spectroscopy is a powerful technique to obtain structural information of membrane proteins immersed in aqueous solution. By exchanging buffer solution with and without salts, the difference spectra between the two conditions provide the structural information relating to the interaction change between membrane proteins and ions, especially around the ion binding site.
V-ATPase from Enterococcus hirae physiologically transports Na+ and Li+ across a hydrophobic lipid bilayer, which forms a large supramolecular protein complex (total molecular weight: ~700,000). Stabilization of these cations in the binding site has been discussed on the basis of X-ray crystal structures of a membrane-embedded domain, the K-ring (Na+ and Li+ bound forms). Here, we applied ATR-FTIR spectroscopy on this large protein complex and measured sodium or lithium ion binding-induced difference infrared spectra of the intact V-ATPase at physiological temperature and under sufficient amount of hydration for the first time. The results suggest that sodium or lithium ion binding induce the deprotonation of Glu139, a hydrogen-bonding change in the tyrosine residue and a little conformational change in the K-ring. These structural changes, especially the deprotonation of Glu139, are considered to be important for reducing energetic barriers to the transport of cations through the membrane.
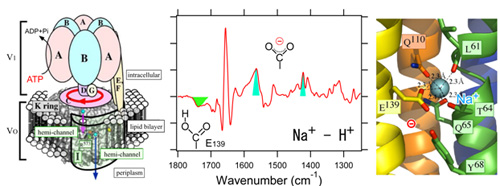
(left) Schematic structure of whole V-ATPase protein complex (center) Sodium ion binding induced difference infrared spectrum. The green and cyan colored bands are assigned to protonated and deprotonted carboxylic acid residues of Glu139 in K-ring, respectively. (right) Sodium ion binding site revealed by X-ray crystallography on K-ring.
Paper Information
Journal: Journal of the American Chemical Society
February 14, 2011 (Communication) Online, DOI: 10.1021/ja1116414
Title: Sodium or Lithium Ion Binding-Induced Structural Changes in the K-ring of V-ATPase from Enterococcus hirae Revealed by ATR-FTIR Spectroscopy
Authors: Yuji Furutani, Takeshi Murata, Hideki Kandori

