
Mixed-valence compounds, typically bearing two bridged metal centers in different formal oxidation state, have played an important role to study electron transfer, which is one of the most fundamental reactions in physics, chemistry and biology. A pivotal issue in mixed-valence chemistry has been the extent of the electronic interaction among multiple redox centers, which determines the efficiency of the intramolecular electron transfer. To clarify the electronic interaction, an absorption resulting from intervalence charge-transfer in the visible or near-infrared region is of prime importance as a systematic marker. However, it has been a very difficult task to address this issue experimentally, and theoretical calculations have been frequently employed as the only available tool in previous studies. Recently, Dr. Takuya Kurahashi and Prof. Hiroshi Fujii succeeded in an unambiguous assignment of the IVCT bands, resulting from mixed-valence state for manganese(III) and nicket(II) salen mono-phenoxyl radical complexes, by using nonsymmetrical salen complexes. This study enables us to obtain otherwise inaccessible insight into the mixed-valence property.
According to the extent of the electronic interaction, mixed-valence compounds are classified into three categories, Class I, II and III, as proposed by Robin and Day. In Class I mixed-valence compounds, no electron transfer occurs, when two bridged metal centers are far apart or when their interaction is symmetry or spin forbidden. In Class II mixed-valence compounds, two metal centers have detectable electronic interaction, and electron transfer is occurring between two metal centers. In Class III mixed-valence compounds, two metal centers have such strong electronic interaction that delocalization occurs, and the formal charge on each metal center is averaged. Class III mixed-valence compounds are distinct from Class II in delocalization of the unpaired electron as a consequence of disappearance of the activation barrier for electron transfer. In addition to the Robin-Day classification, a number of recent studies have reported complicated systems exhibiting an intermediate behavior between Class II and Class III, which are now categorized as borderline Class II–III. Ligand radicals from salen complexes are unique mixed-valence compounds, in which a phenoxyl radical is electronically linked to a remote phenolate via a neighboring redox-active metal ion, providing an opportunity to study electron transfer from a phenolate to a phenoxyl radical mediated by a redox-active metal ion as a bridge (Figure 1).
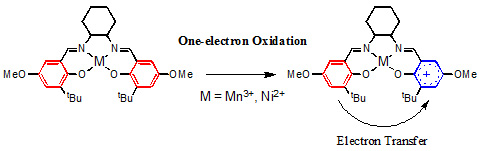
Figure 1. Mixed-valence system formed from one-electron oxidation of manganese(III) and nickel(II) salen complexes.
We synthesized one-electron oxidized products from electronically-diverse manganese(III) salen complexes, in which the locus of oxidation is shown to be ligand-centered, not metal-centered, affording manganese(III)–phenoxyl radical species. As shown in Figure 2 (black line), the MnIII(salen+•)(OTf)2 complex exhibits intense absorptions at 435 nm, which could be assigned as π–π* transitions of the coordinated phenoxyl radical. A mixed-valence property of MnIII(salen+•)(OTf)2 complex can be seen in an absorption feature in the visible and near-infrared region. The MnIII(salen+•)(OTf)2 complex exhibits characteristic broad absorptions at 1270 nm. In order to unambiguously assign this absorption, we measured the absorption spectra of MnIII(salen+•)(OTf)2 from nonsymmetrical manganese(III) salen complexes, MnIII(L-OMe/t-Bu) and MnIII(L-OMe/Cl) (Figure 2). The MnIII(salen+•)(OTf)2 complexes from nonsymmetrical MnIII(L-OMe/t-Bu) and MnIII(L-OMe/Cl), in which one of the MeO group in MnIII(L-OMe) is replaced by t-Bu and Cl, show broad absorptions of similarly low intensity at 1015 and 890 nm, respectively (Figure 2, blue and red lines). The absorptions shifted to a higher energy are consistent with the assignment as a charge transfer band from the phenolate to the phenoxyl radical, because an excitation energy required for photo-induced intramolecular electron transfer would increase in the order L-OMe < L-OMe/t-Bu < L-OMe/Cl. It is noted the ligand-to-ligand charge transfer is exactly the IVCT band from a localized Robin-Day Class II mixed-valence system, and then the MnIII(salen+•)(OTf)2 complex from MnIII(L-OMe) are unambiguously assigned as a Class II mixed-valence compound.
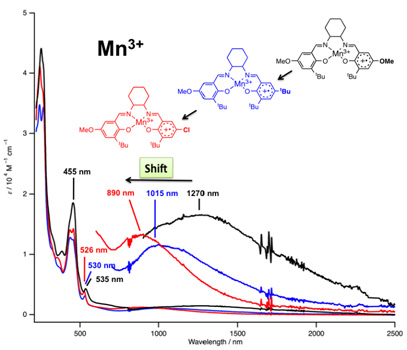
Figure 2. Absorption spectra of manganese(III) salen phenoxyl radical complexes.
Our methodology using a series of nonsymmetrical salen complexes was also applied for the analysis of the NiII(salen+•) complex. One of the best evidence for this assignment is an intense near-infrared absorption at 2140 nm (see black line in Figure 3). A new finding we have made herein was that the NiII(salen+•) complex from NiII(L-OMe) exhibits a significantly broad absorption in the range from 1200 to 2000 nm, in addition to a narrow absorption at 2140 nm, indicative of its different mixed-valence property. The narrow absorption at 2140 nm can be assigned as a delocalized Class III IVCT band, in comparison with the absorption spectrum of NiII(salen+•) from NiII(L-t-Bu), a delocalized Class III mixed-valence compound. This assignment is justified by complete disappearance of the narrow near-infrared absorption in the NiII(salen+•) complex from nonsymmetrical NiII(L-OMe/t-Bu) and NiII(L-OMe/Cl) (see blue and red lines in Figure 3), in which the radical should be localized on the methoxyphenolate moiety due to the difference of the redox potential. In contrast, the broad absorption at ~1950 nm in NiII(salen+•) from NiII(L-OMe), which is shifted to a higher energy (1580 and 1400 nm) in NiII(salen+•) from nonsymmetrical NiII(L-OMe/t-Bu) and NiII(L-OMe/Cl), is reliably assigned as a Class II charge transfer transition. It is clearly shown the NiII(salen+•) complex from NiII(L-OMe) is just on the transition between Class II and Class III mixed-valence compounds. Consistently, the NiII(salen+•) complex from NiII(L-OMe) shows an EPR signal (S = 1/2) at g = 2.023, which is close to the EPR signals at g = 2.021 and 2.016 for localized NiII(salen+•) from nonsymmetrical NiII(L-OMe/t-Bu) and NiII(L-OMe/Cl), as compared to the EPR signal at g = 2.045 for delocalized NiII(salen+•) from NiII(L-t-Bu).
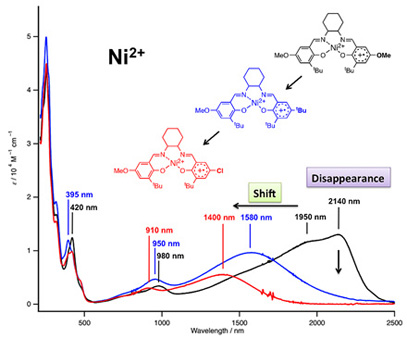
Figure 3. Absorption spectra of nickel(II) salen phenoxyl radical complexes.
The present findings point to a fascinating possibility that electron transfer could be drastically modulated by exchanging the metal ion that bridges the two redox centers, or by adjusting the redox potential of the electron donor and acceptor relative to the metal ion mediator.
Paper Information
Journal:Journal of the American Chemical Society, Publication Date (Web): May 10, 2011 (Article), DOI: 10.1021/ja2016813
Title: One-Electron Oxidation of Electronically Diverse Manganese(III) and Nickel(II) Salen
Complexes: Transition from Localized to Delocalized Mixed-Valence Ligand Radicals
Authors: Takuya Kurahashi and Hiroshi Fujii
FUJII Group Information
http://www.ims.ac.jp/english/know_en/bio/fujii/fujii_en.html



