Antibody binds Fc receptor not only with its Fc trunk but also through its Fab arm.
English version 21 August, 2019 on EurekAlert!
Antibodies are key players in our immune system and have been used as biopharmaceuticals. The collaborative groups including researchers at Nagoya City University, National Institutes of Natural Sciences (ExCELLS/IMS), and Osaka University have found previously unknown contact sites in the antibody molecule that are involved in its binding to a cognate receptor, challenging the traditional paradigm of the molecular mechanism of antibody function. Their findings have been published in Scientific Reports.
In our immune system, antibodies recognize viruses, bacteria and even cancer cells through their Fab arms, initiating recruitment of leucocytes for the destruction of these invaders. The recruitment is mediated by receptors on the leucocytes which, to date, have been supposed to bind the Fc portion of the antibody and have therefore been termed Fc receptors. This textbook view has been established based on cumulative data obtained primarily using Fab and Fc fragments cleaved from antibody molecules.
The collaborative groups including researchers at Nagoya City University, National Institutes of Natural Sciences (ExCELLS/IMS), and Osaka University reassessed this paradigm employing modern biophysical techniques. Using high-speed atomic force microscopy and hydrogen–deuterium exchange mass spectrometry, they revealed that, in addition to the canonical Fc-mediated contact, the Fab portion of the antibody is directly involved in binding to a cognate Fc receptor.
Enhancement of Fc receptor binding by molecular engineering is a promising strategy to improve the functional efficacy of therapeutic antibodies for cancer treatments. Such attempts have thus far mainly focused on the Fc trunk of the antibody. The groups’ findings will open up a new avenue for developing therapeutic antibodies with enhanced binding properties to the Fc receptor by engineering approaches targeting the previously unknown contact sites in their Fab arms.
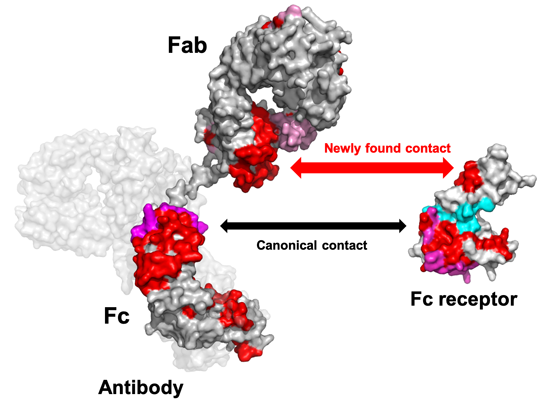 Fig. Mapping of the Newly Found Contact Sites between Antibody and Fc Receptor
Fig. Mapping of the Newly Found Contact Sites between Antibody and Fc Receptor
VIDEO: Antibody (pink arrow) transiently interacts with Fc receptor (white arrow) immobilized on the mica surface.
Journal
Journal: Scientific Reports
Title:
The Fab portion of immunoglobulin G contributes to its binding to Fcγ receptor III
Author:
Rina Yogo, Yuki Yamaguchi, Hiroki Watanabe, Hirokazu Yagi, Tadashi Satoh, Mahito Nakanishi, Masayoshi Onitsuka, Takeshi Omasa, Mari Shimada, Takahiro Maruno, Tetsuo Torisu, Shio Watanabe, Daisuke Higo, Takayuki Uchihashi, Saeko Yanaka, Susumu Uchiyama & Koichi Kato
DOI: 10.1038/s41598-019-48323-w
Contact
Koichi KATO
Graduate School of Pharmaceutical Sciences, Nagoya City University
(Exploratory Research Center on Life and Living Systems/Institute for Molecular Science)
3-1 Tanabe-dori, Mizuho-ku, Nagoya 467-8603, Japan
TEL:+81-52-836-3447
FAX:+81-52-836-3447
E-mail:kkato_at_phar.nagoya-cu.ac.jp (Please replace the "_at_" with @)
1120

 Fig. Mapping of the Newly Found Contact Sites between Antibody and Fc Receptor
Fig. Mapping of the Newly Found Contact Sites between Antibody and Fc Receptor