Research Paper entitled "Thickness dependent homogeneous crystallization of ultrathin amorphous solid water films" published on January 3, 2020 by research group including Kuniaki Harada, graduate student at Kyoto University, and Toshiki Sugimoto, associate professor at the Institute for Molecular Science, has been selected for “2020 PCCP HOT Articles” in Physical Chemistry Chemical Physics (PCCP).
This paper is available for free access until the end of June 2020 and can be viewed at the following URL.
https://pubs.rsc.org/en/content/articlelanding/2020/cp/c9cp05981d#!divAbstract
Outline of this paper
The crystallization of amorphous materials is generally triggered by spontaneous creation of crystalline nuclei. Two processes are distinguishable in terms of the place where crystallized nuclei are formed: heterogeneous nucleation at the surface of material or the interface with the other material, and homogeneous nucleation in the bulk. In general, the surface and interface of amorphous thin films serve as nucleation sites. It has been traditionally believed that crystallization of amorphous ice thin films also proceeds via the heterogeneous nucleation.
We have focused on the ultrathin films of amorphous ice grow on Pt(111) substrate and systematically investigated the crystallization process by varying the thickness of the films from a few nm to several tens of nm. Simultaneously monitoring the crystallization processes at the surface and in the interior of ice films, we found that the crystallization proceeds via homogeneous nucleation irrespective of the film thickness. This discovery overturned the conventional idea that the crystallization of amorphous ice thin films is initiated by heterogeneous nucleation. Furthermore, we found that the crystallization kinetics and temperature of amorphous ice thin films are highly modulated depending on the thickness of the film, although crystallization itself proceeds via the homogeneous nucleation mechanism (Fig. 1). The structural analysis of hydrogen bonds based on vibrational spectroscopy revealed that the strength of hydrogen bonds in the thermodynamically most relaxed amorphous ice films (the state just before crystallization) changes significantly with the film thickness (Fig. 1), which shows an evidence of a peculiar size effect.
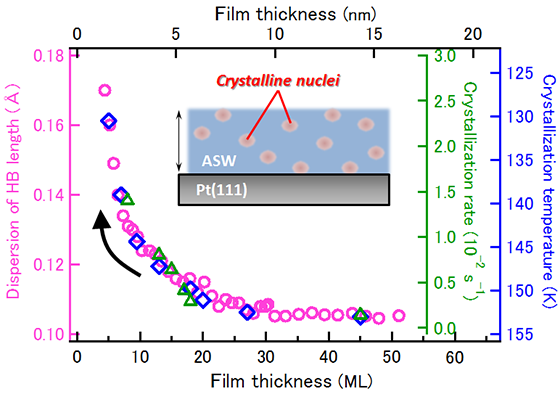
Fig. 1: Thickness dependence of the distribution of O-H…O hydrogen bond length (○), crystallization rate (△), and crystallization temperature (◇) of amorphous ice thin films on Pt(111). Crystallization proceeds homogeneously across the films.
The crystallization mechanism and kinetics are fundamentally important for thermal stability of amorphous materials. It has often been attempted to control the thermal stability of ultrathin films of amorphous materials by modulating the microscopic structure at surfaces and interfaces. However, this study shows that modulating the "thickness" of the film itself can be essential for the design and control of the stability of amorphous thin films. For future prospect, we are also interested in systematic understanding of the crystallization behavior of amorphous ice films on other substrates, and also in changes in the crystallization behavior in the much thicker films.
3971

