English version 1 April, 2021 on EurekAlert!
An international team of researchers has synthesized curved, infinitely stacking nanographenes -- like potato chips in a cardboard can -- that can assemble into nanowires.
-------------------------------------------------------------------------
Nanographene is flexible, yet stronger than steel. With unique physical and electronic properties, the material consists of carbon molecules only one atom thick arranged in a honeycomb shape. Still early in technological development, current fabrication methods require the addition of substituents to obtain a uniform material. Additive-free methods result in flimsy, breakable fibers--until now.
An international team of researchers has developed self-assembling, stable and strong nanographene wires. The results were published on March 24 in Journal of the American Chemical Society.
The team, led by Yasutomo Segawa, associate professor at the Institute for Molecular Science, part of the National Institutes of Natural Science in Japan, set out to synthesize curved, infinitely stacking nanographenes -- like potato chips in a cardboard can -- that can assemble into nanowires.
"Effectively stacked hydrocarbon wires have the potential to be used as a variety of nano-semiconductor materials," Segawa said. "Previously, it has been necessary to introduce substituents that are not related to or inhibit the desired electronic function in order to control the assembly of the wires."
By removing substituents, or additives, from the fabrication process, researchers can develop molecular materials that have a specific, desired electronic function, according to Segawa. With this goal in mind, the team developed a molecule called 'bitten' warped nanographene (bWNG), with 68 carbon atoms and 28 hydrogen atoms forming a 'bitten apple' shape. Created as a solution, when left to evaporate over 24 hours in the presence of hexane -- an ingredient in gasoline with six carbon atoms -- bWNG becomes a gel.
The researchers attempted to recrystallize the molecules of the original solution to examine the specific structure of the bWNG gel through X-ray crystallography. This technique can reveal the atomic and molecular structure of a crystal by irradiating the structure with X-rays and observing how they diffract.
"We attempted recrystallizing many times to determine the structure, but it grew to only a few hundred nanometers," Segawa said, noting that this size is much too small for X-ray crystallography. "It was only by electron diffraction, a new method for determining the structure of organic materials, that we were able to analyze the structure."
Electron diffraction is similar to X-ray crystallography, but it uses electrons instead of X-rays, resulting in a pattern of interference with the sample material that indicates the internal structure.
They found that the bWNG gel consisted of double-stranded, double-helix nanofibers that assembled themselves from curved, stackable nanographenes.
"The structure of the nanofibers is a double-stranded double helix, which is very stable and, therefore, strong," Segawa said. "Next, we would like to realize a semiconductor wire made entirely of carbon atoms."
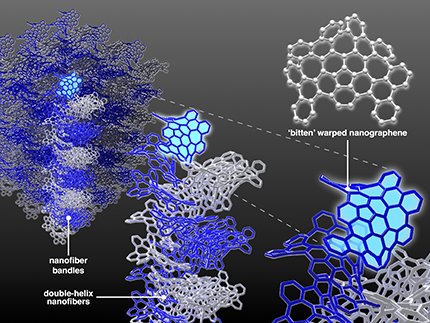
Fig.1 Schematic illustration of hierarchical structures of carbon nanofiber bundles made of bitten warped nanographene molecules
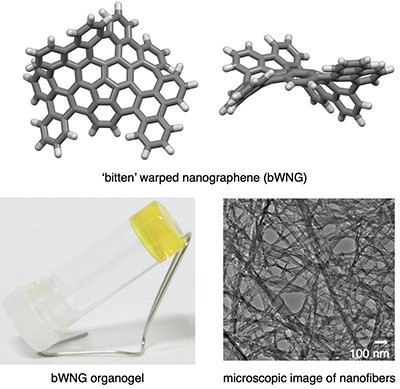
Fig.2 Upper panel shows the molecular structure of 'bitten' warped nanographene (bWNG). Lower left shows a photograph of bWNG organogel and Lower right shows a microscopic image of nanofibers made of bWNG.
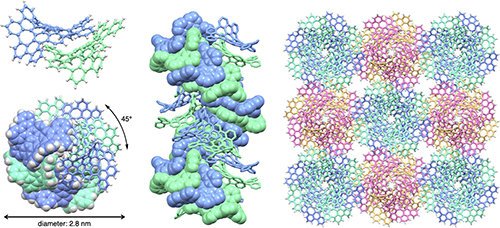
Fig.3 Structure of double-helix supramolecular nanofibers assembled from 'bitten' warped nanographenes (bWNG). (Upper left) An assembly of two bWNGs. (Lower left) Top view of a nanofiber. A double-helix with a diameter of 2.8 nm is formed with each molecule shifted by 45 degrees. (Middle) Side view of a nanofiber. (Right) Nanofiber bundles.
Co-authors include Kenta Kato, Nobuhiko Mitoma, Taishi Nishihara, Yuh Hijikata and Kenichiro Itami, along with Segawa, with the Graduate School of Science, Nagoya University; Kiyofumi Takaba, Saori Maki-Yonekura and Koji Yonekura, Biostructural Mechanism Laboratory, RIKEN; Yusuke Nakanishi, Graduate School of Science, Tokyo Metropolitan University; Taito Hatakeyama and Takuma Kawada, Central Research Laboratory Technology and Development Division, Kanto Chemical Co., Inc.; and Lawrence T. Scott, Department of Chemistry, University of Nevada. Segawa, Mitoma, Nishihara and Itami are also affiliated with the Itami Molecular Nanocarbon Project at Nagoya University. Nishihara is also affiliated with the Institute of Advanced Energy at Kyoto University. Mitoma is also affiliated with the RIKEN Center for Emergent Matter Science. Hijikata, along with Itami, is also affiliated with the Institute of Transformative Bio-Molecules at Nagoya University with co-author Jenny Pirillo. Hijikata and Pirillo are also affiliated with the Institute for Chemical Reaction Design and Discovery at Hokkaido University. Yonekura is also affiliated with the Advanced Electron Microscope Development Unit, RIKEN-JEOL Collaboration Center and Institute of Multidisciplinary Research for Advanced Materials, Tohoku University. Segawa is also affiliated with the Department of Structural Molecular Science, The Graduate University for Advanced Studies.
The Japan Science and Technology Agency, the Ministry of Education, Culture, Sports, Science and Technology, the Japan Society for the Promotion of Science, the Toyoaki Scholarship Foundation, the Daiko Foundation and the United State National Science Foundation funded this work.
Information of the paper
Authors: Kenta Kato, Kiyofumi Takaba, Saori Maki-Yonekura, Nobuhiko Mitoma, Yusuke Nakanishi, Taishi Nishihara, Taito Hatakeyama, Takuma Kawada, Yuh Hijikata, Jenny Pirillo, Lawrence T. Scott, Koji Yonekura, Yasutomo Segawa, and Kenichiro Itami
Journal Name: Journal of the American Chemical Society
Journal Title: Double-helix supramolecular nanofibers assembled from negatively curved nanographenes
DOI: 10.1021/jacs.1c00863
Financial Supports
Japan Science and Technology Agency (JPMJER1302, JPMJMI20G5)
Grant-in-Aid for Scientific Research (JP19H05463, JP19H02701, JP17H05149)
Japan Agency for Medical Research and Development
Daiko Foundation
Toyoaki Scholarship Foundation
Contact Person
Yasutomo Segawa
TEL: +81-564-59-5587
E-mail: segawa_at_ims.ac.jp (Please replace the "_at_" with @)
4621



