Non-thermal methane activation and conversion under ambient conditions hold immense promise for sustainable use of methane resources. However, incomplete knowledge on microscopic reaction mechanisms hampers the development of engineering strategies for the reaction system. Here, combining real-time mass spectrometry and operando infrared absorption spectroscopy with ab initio molecular dynamics simulations, the crucial role of interfacial water in the non-thermal C–H activation during photocatalytic methane conversion was discovered.
-------------------------------------------------------------------
Very recently, researchers led by Toshiki Sugimoto, Associate Professor at the Institute for Molecular Science, succeeded in obtaining key molecular-level insights into the crucial role of interfacial water on the non-thermal C–H activation in photocatalytic methane conversion. Combining real-time mass spectrometry and operando infrared absorption spectroscopy with ab initio molecular dynamics simulations, they showed that the methane conversion is hardly induced by the direct interaction with the trapped hole at the surface Olat site; instead, activation is significantly promoted by low barrier hydrogen abstraction from methane by the photoactivated interfacial water species (Figure 1, 2). In the water-mediated processes, the photocatalytic C–H activation is not the rate-determining step, which is in stark contrast to the case of traditional thermocatalytic methane reforming. Moreover, owing to the moderate stabilization of •CH3 in the hydrogen-bond network of water (Figure 3), the overall photocatalytic conversion rates are dramatically improved by typically more than 30 times at ambient temperatures (~300 K) and pressures (~1 atm) (Figure 1). As essentially opposed to thermal catalysis, methane photocatalysis no longer requires high-pressure methane gas (> 20 atm) in the presence of adsorbed water layer.
The water-assisted effects are noticeable also in ethane formation, although water is not explicitly involved in the homocoupling reaction equation (2CH4 → C2H6 + H2). These results indicate that the interfacial water kinetically plays crucial roles beyond the traditional thermodynamic concept of redox potential, in which oxidation of water by surface trapped holes is less thermodynamically favored than methane oxidation: E°•OH/H2O = 2.73 V and E°•CH3/CH4 = 2.06 V versus the standard hydrogen electrode. Notably, these water-assisted effects are commonly observed for several representative photocatalysts with the different band-gap energy, such as TiO2, Ga2O3, and NaTaO3, indicating that the incorporation of methane into the photoactivated interfacial hydrogen-bond network is essential key for the non-thermal activation of methane.
Our work not only expands the molecular-level understanding of the non-thermal C–H activation and conversion but also provides a fundamental basis for the rational interface design of non-thermal catalytic systems toward the effective and sustainable utilization of methane under ambient conditions.
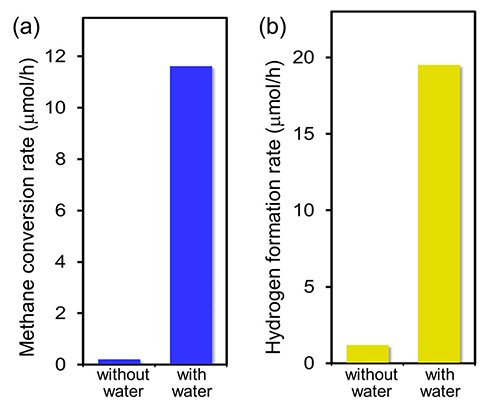
Figure 1: Effect of water
(a) Methane conversion rates and (b) hydrogen formation rates on the Pt/Ga2O3 photocatalysts under ultraviolet irradiation at a methane partial pressure of 70 kPa and water partial pressures of 0 and 2 kPa at a sample temperature of 318 K. The presence of interfacial water significantly enhances the photocatalytic activity at ambient temperatures and pressures.
 aaaaaa
aaaaaa
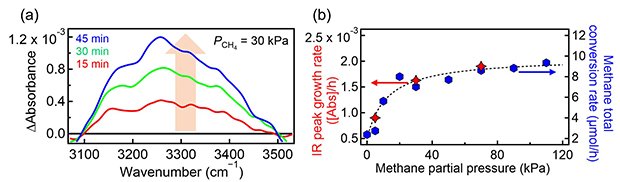
Figure 2: Operando IR spectra
(a) Time evolution of operando infrared (IR) spectra in the O–H stretching region for Pt/Ga2O3 photocatalysts under ultraviolet irradiation at a CH4 pressure of 30 kPa and a D2O pressure of 2 kPa. The O–H peak growth indicates the hydrogen abstraction on catalyst surfaces by photoactivated interfacial water species (CH4(gas) + •OD(ad) → •CH3(ad) + HDO(ad)). (b) Growth rate of the O–H peak (left axis) and CH4 total conversion rate (right axis) on the Pt/Ga2O3 photocatalysts as functions of methane partial pressure; there is a good correlation between the two plots.
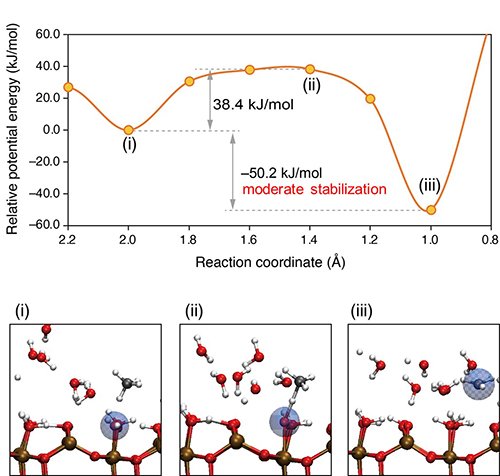
Figure 3: AIMD results
Ab-initio molecular dynamics (AIMD) simulation results. Potential energy curve (PEC) and snapshots corresponding to the dots on PEC (i–iii) for methane activation with water on a Ga2O3 surface. The blue-shaded spheres in the snapshots indicate the hole, which is identified from a Mulliken spin population value larger than 0.5 e. (i) a methane molecule in gas phase (ii) interacts with a photoactivated interfacial water molecule and (iii) a photoactivated •CH3 intermediate is formed with moderate stabilization (~50 kJ/mol).
Information of the paper
Authors: Hiromasa Sato, Atsushi Ishikawa, Hikaru Saito, Taisuke Higashi, Kotaro Takeyasu, and Toshiki Sugimoto
Journal Name: Communications Chemistry
Journal Title: “Critical impacts of interfacial water on C–H activation in photocatalytic methane conversion”
DOI: 10.1038/s42004-022-00803-3
Financial Supports
JST-PRESTO, JPMJPR16S7
JSPS KAKENHI
Grant-in-Aid for Specially Promoted Research, 17H06087
Grant-in-Aid for Scientific Research (A), 19H00865
Grant-in-Aid for JSPS Fellow, 22J13055
Joint Research by the National Institutes of Natural Sciences (NINS), 01112104
Demonstration Project of Innovative Catalyst Technology for Decarbonization through Regional Resource Recycling, the Ministry of the Environment, Government of Japan
Contact Person
Toshiki Sugimoto
TEL: +81-564-55-7280
E-mail: toshiki-sugimoto_at_ims.ac.jp (Please replace the "_at_" with @)
3971


 aaaaaa
aaaaaa