Selective methane oxidation to desirable chemicals under ambient conditions is a key area of research in catalytic chemistry. However, control of non-thermal methane oxidation remains a challenge due to the lack of microscopic insight into the reaction mechanisms. Here, the novel photocatalytic roles of metal cocatalysts as hole acceptors and promoters of oxidation reactions were unveiled by operando infrared absorption spectroscopy. This new understanding provides a solid basis for controlling hole-driven oxidation through metal-cocatalyst engineering.
The photocatalytic conversion of methane with a ubiquitous and clean oxidant of water has the potential to develop into an on-site and on-demand chemical technology for the green utilization of methane in an environmentally benign and sustainable way. However, rational design of next-generation photocatalysts is hindered by the lack of molecular-level understanding of hole-driven oxidation kinetics, active sites, and resultant photocatalytic performance.
The research group led by Toshiki Sugimoto, Associate Professor at the Institute for Molecular Science, has demonstrated that metal cocatalysts loaded on a semiconductor photocatalyst play critical roles in modulating surface oxidation kinetics and resultant oxidation selectivity. Real-time mass spectrometric analysis of gaseous products under systematically-controlled methane pressures revealed that the Pt-loaded Ga2O3 photocatalyst predominantly promoted the total oxidation of methane toward CO2 on its surface, while the Pd-loaded photocatalyst exhibited a higher selectivity for C2H6 formation through the gas-phase coupling of free •CH3 (Figure 1). This difference in methane oxidation kinetics was corroborated by operando infrared absorption spectroscopy that observed surface intermediates under working conditions (Figure 2). Moreover, the research group demonstrated that the Pt cocatalyst itself was oxidized by photogenerated holes. These experimental results demonstrated the critical roles of metal cocatalysts as a reservoir of photogenerated holes and an effective reaction site for methane oxidation processes.
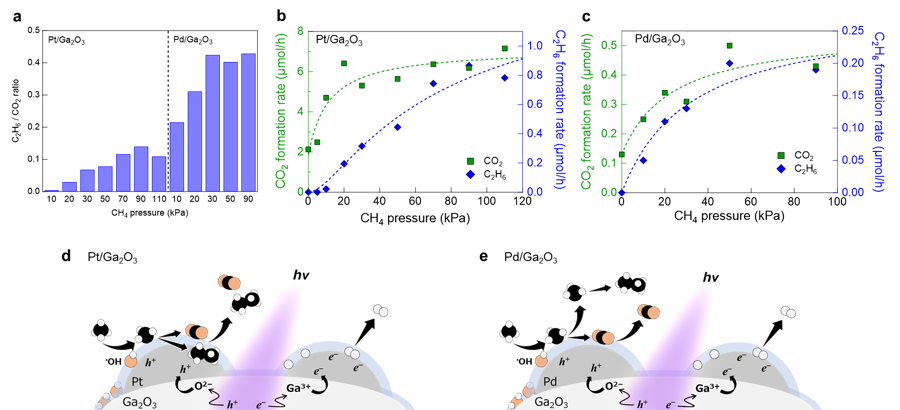
Figure 1: Photocatalytic performance and reaction kinetics. (a) The C2H6 to CO2 ratios of Pt/Ga2O3 and Pd/Ga2O3 photocatalysts. The Pd cocatalyst promotes C2H6 formation more efficiently than the Pt cocatalyst. Methane pressure dependence on the formation rates of CO2 and C2H6 over (b) Pt/Ga2O3 and (c) Pd/Ga2O3 photocatalysts at ~323 K. The pressure of water vapor was fixed at 2 kPa. Schematic illustration of photocatalytic methane oxidation and reduction taking place on (d) Pt and (e) Pd cocatalysts covered with a single water layer.
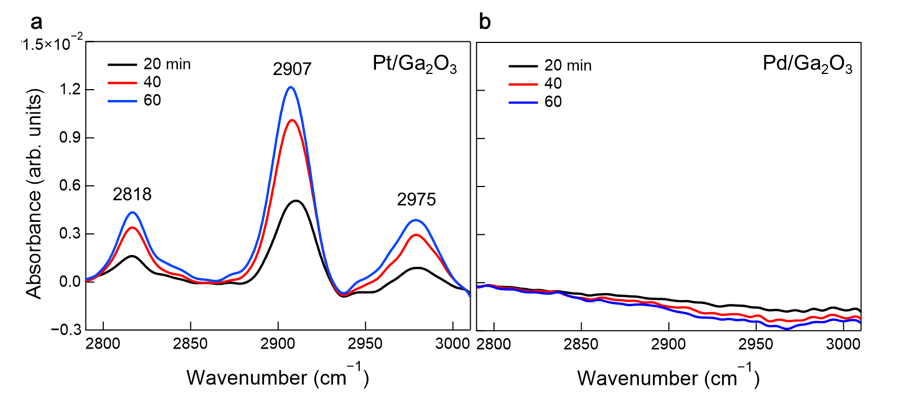
Figure 2:Operando infrared absorption spectra. Time evolution of operando infrared spectra in the C–H stretching region for (a) Pt/Ga2O3 and (b) Pd/Ga2O3 photocatalysts under ultraviolet light irradiation at methane and water pressure of 30 and 2 kPa, respectively. The appearance of the C–H peaks on Pt/Ga2O3 demonstrates methane oxidation taking place on its surface. No C–H peaks on Pd/Ga2O3 indicates the low population of the hydrocarbon intermediates due to the desorption of the methyl radical intermediate.
Generally, metal cocatalysts have been recognized for half a century as reduction cocatalysts that exclusively accumulate photogenerated electrons and promote reduction reactions such as H2 evolution. Based on this conventional assumption, hole-accumulated metal cocatalysts are assumed to act as charge recombination centers and inhibit photocatalysis. In contrast, this research group verified that both H2 evolution and methane oxidation were accelerated by metal cocatalyst loading. These experimental results indicate that photogenerated electrons and holes are separately trapped at different metal cocatalyst particles while avoiding charge recombination and promoting redox reactions (Figure 1d, e).
Thus, the systematic operando investigation of the photocatalytic oxidation of methane (i.e., the most inert and simplest organic compound) and water (i.e., one of the key molecules in photocatalysis) provides a new paradigm for the role of metal cocatalysts in photocatalysis and thus contributes to developing a cocatalyst-based surface engineering strategy for controlling non-thermal oxidation reactions.
Paper Information
Authors: Hikaru Saito, Hiromasa Sato, Taisuke Higashi, and Toshiki Sugimoto
Journal Name: Angewandte Chemie International Edition (Angewandte Chemie)
Journal Title: “Beyond Reduction Cocatalysts: Critical Role of Metal Cocatalysts in Photocatalytic Oxidation of Methane with Water”
DOI: 10.1002/anie.202306058 (10.1002/ange.202306058)
Financial Support
JST-PRESTO, JPMJPR16S7
JSPS KAKENHI
Grant-in-Aid for Specially Promoted Research, 17H06087
Grant-in-Aid for Scientific Research (A), 19H00865, 22H00296
Grant-in-Aid for JSPS Fellow, 22J13055, 22J01398
Joint Research by the National Institutes of Natural Sciences (NINS), 01112104
Special Project by Institute for Molecular Science (IMS), 22IMS1101
Demonstration Project of Innovative Catalyst Technology for Decarbonization through Regional Resource Recycling, the Ministry of the Environment, Government of Japan
Contact Person
Toshiki Sugimoto
TEL: +81-564-55-7280
E-mail: toshiki-sugimoto_at_ims.ac.jp (Please replace the "_at_" with @)
3971


