Synchronizing periodic excitations of photocatalysts with a Michelson interferometer on operando FT–IR spectroscopy, researchers led by Toshiki Sugimoto succeeded in observing and identifying the reactive electron species for photocatalytic hydrogen evolution. In contrast to the traditional belief, this study demonstrates that not the free electrons in metal cocatalysts but the electrons trapped in the periphery of cocatalysts directly contribute to the photocatalysis.
Since the discovery of photoelectrochemical hydrogen evolution by Honda and Fujishima in 1972, heterogeneous photocatalysis has been intensively investigated and is still a hot topic in science and technology. In particular, understanding of reactive electron species and active reaction sites on photocatalytic reduction reaction are vital for designing and manufacturing innovative catalysts with improved evolution activity of hydrogen as sustainable energy carrier. However, despite its fundamental importance, microscopic understanding on photocatalysis remains a highly challenging issue owing to an inherent difficulty in experimentally observing and extracting weak spectroscopic signals originating from the photoexcited reactive electron species. This is predominantly due to inevitable temperature increment for catalyst samples under actual photocatalytic reaction conditions upon continuous photon irradiation. In this case, the weak signals derived from reactive photoexcited electron species are readily overwhelmed by intense background signals originating from thermally excited nonreactive electrons.
Researchers (Dr. Hiromasa Sato and Prof. Toshiki Sugimoto) at Institute for Molecular Science / The Graduate University for Advanced Studies, SOKENDAI succeeded in significantly suppressing the signals derived from thermally excited electrons and observing the reactive photogenerated electrons contributing to the photocatalytic hydrogen evolution. Such innovation was achieved by a new method based on synchronization of the millisecond periodic excitations of photocatalysts with a Michelson interferometer used for FT–IR spectroscopy. This demonstration was achieved for metal-loaded oxide photocatalysts under steam methane reforming and water splitting conditions. Although it has long been conventionally believed that loaded metal cocatalysts function as sinks for reactive photogenerated electrons and active sites for reduction reactions, they found that the free electron species in the metal cocatalysts were not directly involved in the photocatalytic reduction reaction. Alternatively, the electrons shallowly trapped in the in-gap states of oxides contributed to enhancing the hydrogen evolution rate upon the loading of metal cocatalysts. The electron abundance in the in-gap states, especially metal-induced semiconductor surface states, was clearly correlated to the reaction activity, suggesting that such metal-induced semiconductor surface states formed in the periphery of the metal cocatalyst play key roles in the photocatalytic hydrogen evolution.
These microscopic insights shift a paradigm on the traditionally believed role of metal cocatalysts in photocatalysis and provide a fundamental basis for rational design of the metal/oxide complex interfaces as promising platforms for nonthermal hydrogen evolution. Furthermore, the new approach of operando infrared spectroscopy is widely applicable to other various catalytic reaction systems and materials driven by the aid of photons and/or external electric field/potential. Therefore, the new approach would have great potential to uncover hidden key factors to enhance catalyst performance toward innovation of environmentally friendly energy technology for next-generation sustainable society.
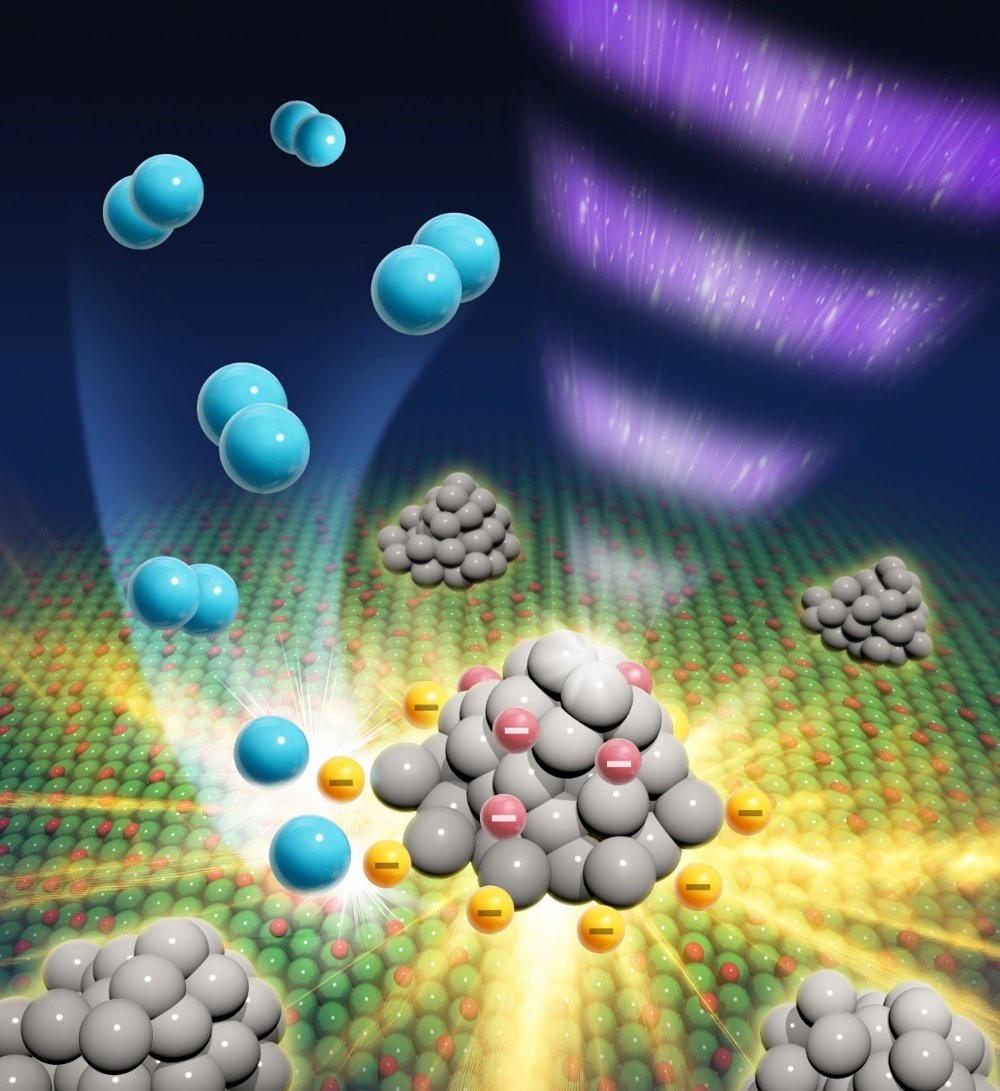
Figure 1:
Schematic illustration of how the photogenerated electrons contribute to photocatalytic H2 evolution reaction on metal-loaded oxides. Not the free electrons in metal cocatalysts but the electrons trapped in the periphery of cocatalysts directly contribute to the photocatalysis. This discovery was achieved by synchronizing periodic excitations of photocatalysts with a Michelson interferometer on operando FT–IR spectroscopy. (Credit: Shinichiro Kinoshita <https://kinoshita-design.com/>, License: This figure is for research use only. Any other use, including commercial purposes, is prohibited.)
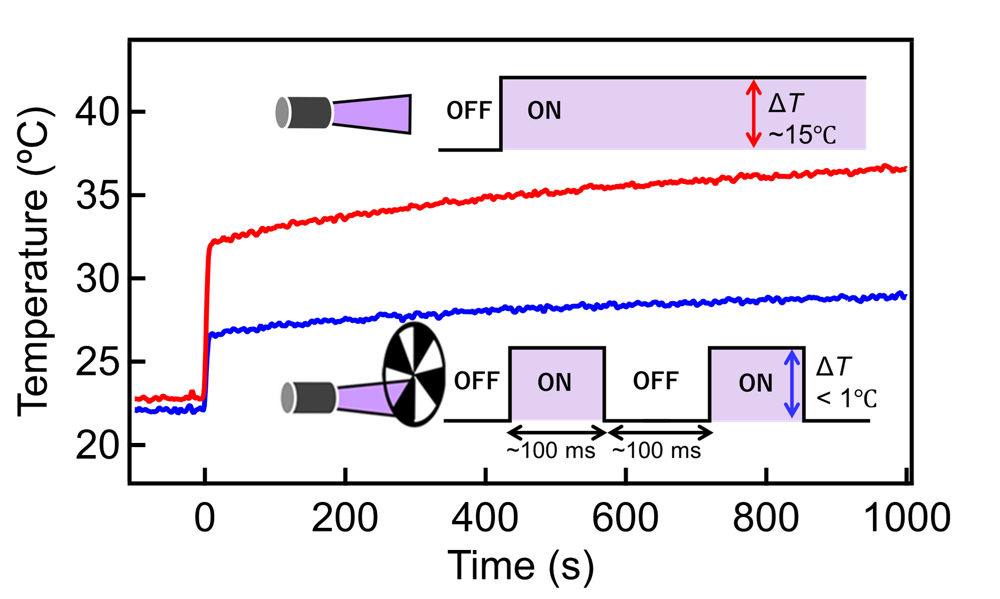
Figure 2: Effect of chopping
Typical time profiles of the net temperature of the Pt/Ga2O3 photocatalyst particles subjected to (red line) continuous UV irradiation (∼90 mW cm–2) and (blue line) periodic UV irradiation with ∼5 Hz modulation at a partial pressure of methane gas (PCH4) of 30 kPa and a partial pressure of water vapor (PH2O) of 2 kPa. Inset: Schematic of the continuous and periodic UV irradiation. During periodic UV irradiation, excitation light irradiation (ON) and nonirradiation (OFF) are periodically repeated by an optical chopper; this significantly suppresses the increase in the instantaneous temperature of the sample. (Credit: Hiromasa Sato, Toshiki Sugimoto, License: CC BY)
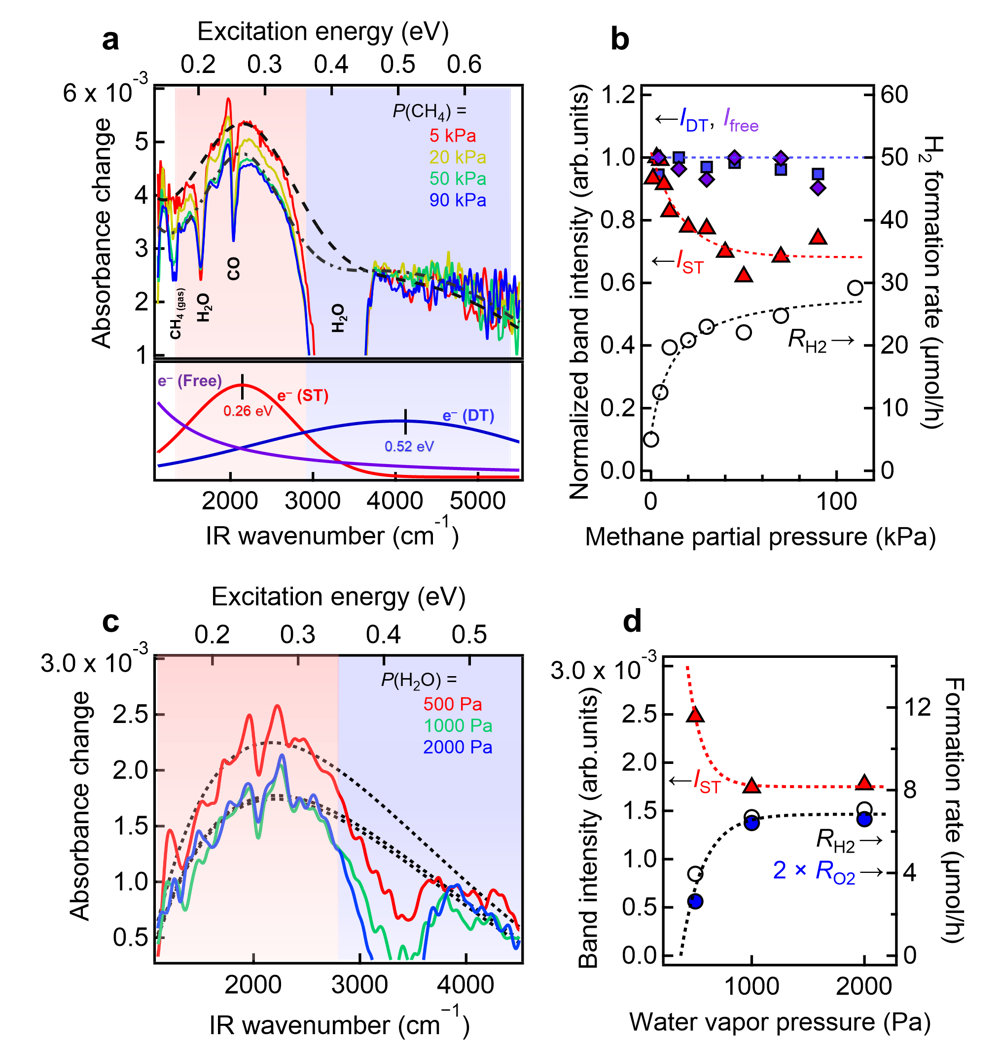
Figure 3: Operando IR spectra
Operando IR spectra of electron species and correlations between the band intensity and formation rates of gaseous products. (a) Operando IR absorbance-change spectra of the Pt/Ga2O3 samples measured under steam methane reforming at PH2O = 2 kPa and different PCH4 values. Typical IR spectra of free electrons (purple), electrons in shallowly trapped (ST) states (red), and electrons in deeply trapped (DT) states (blue) are shown in the bottom panel. (b) Intensities of absorption bands corresponding to free electrons (Ifree), ST electrons (IST), and DT electrons (IDT) as a function of PCH4. PCH4 dependence of the H2 formation rate is shown on the right axis. (c) Operando IR absorbance-change spectra of the Pt/Ga2O3 samples measured under water splitting in the presence of small amount of methane gas (5 kPa). (d) Intensities of absorption bands corresponding to ST electrons (IST) as a function of PH2O. PH2O dependence of the formation rates of H2 and O2 derived from water splitting reaction is shown on the right axis. (Credit: Hiromasa Sato, Toshiki Sugimoto, License: CC BY)
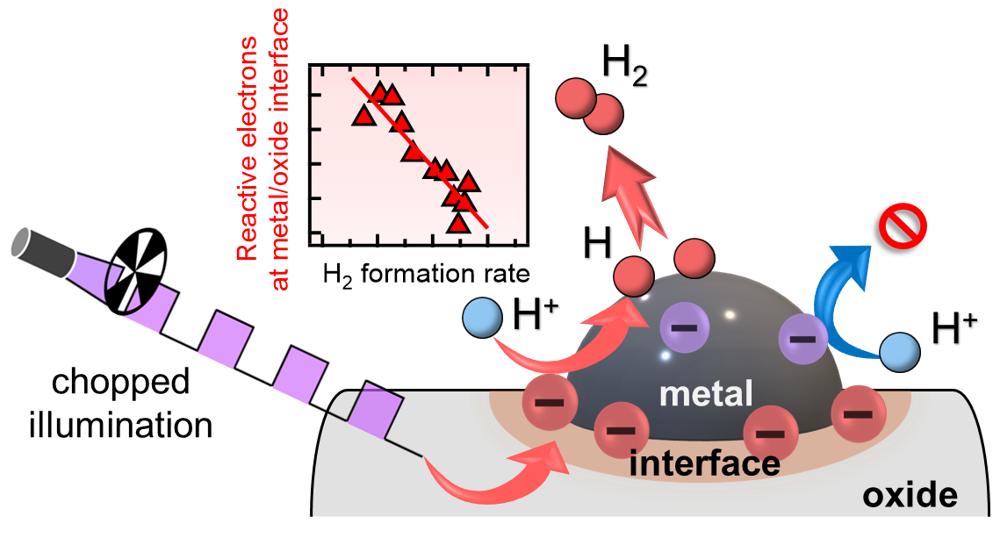
Figure 4: Hydrogen evolution
Schematic illustration of how the ST electrons contribute to photocatalytic H2 evolution reaction. This reaction is initiated by the reduction of protons by the ST electrons on the metal-induced semiconductor surface states at the periphery of the metal cocatalysts. The H atoms produced in the reduction reaction diffuse on the metal cocatalyst and associate to evolve H2 molecules. (Credit: Hiromasa Sato, Toshiki Sugimoto, License: CC BY)
Information of the paper:
Authors: Hiromasa Sato and Toshiki Sugimoto
Journal Name: Journal of the American Chemical Society
Journal Title: “Direct Operando Identification of Reactive Electron Species Driving Photocatalytic Hydrogen Evolution on Metal-loaded Oxides”
DOI: 10.1021/jacs.3c14558
Financial Supports:
JST-PRESTO, JPMJPR16S7
JST-CREST, JPMJCR22L2
JSPS KAKENHI
Grant-in-Aid for Scientific Research (A), JP22H00296
Grant-in-Aid for JSPS Fellows, JP22KJ1427
Grant-in-Aid for Transformative Research Areas (A), JP24H02205
Joint Research by the National Institutes of Natural Sciences (NINS), 01112104
Demonstration Project of Innovative Catalyst Technology for Decarbonization through Regional Resource Recycling, the Ministry of the Environment, Government of Japan
Contact Person:
Name: Toshiki Sugimoto
TEL: +81-564-55-7280
E-mail: toshiki-sugimoto_at_ims.ac.jp (Please replace the "_at_" with @)
3971




