Research Theme
Design, Synthesis, and Functionalization of Chiral Molecules
Keywords
Organic Synthesis, Molecular Catalyst, Non-Covalent Interaction
"Chirality" is a special property that a substance cannot be superimposed with its mirror image, and a molecule with such a property is called "chiral molecule." "Chirality" is known as a factor that advances the properties of various substances. Incorporating chirality into substances leads to a dramatic improvement in their function and is the first step to create dream substances. We are working on creating the fundamental research with the ultimate goal of application to the development of chiral functional materials. We are designing our own chiral molecule, developing our own synthetic method toward its synthesis, and aiming at creating new functions of uniquely synthesized chiral molecules.
We have successfully developed a chiral molecular catalyst with multiple hydrogen bond donor sites. This chiral molecule takes only one specific conformation out of multiple conformations. In addition, we found that this chiral molecule functions as a molecular catalyst to supply chiral small molecules. This result suggests that the function of enzymes with molecular weights of several thousands to tens of thousands can be easily realized by artificial chiral molecules with molecular weights of several hundreds.
We believe that our challenges create chiral molecules, which have new possibilities by designing chiral molecules and making use of the developed reactions. We are planning to elucidate the unknown behavior of synthesized molecules by using various analytical methods and to create chiral substances with new functions by finding properties peculiar to molecules.
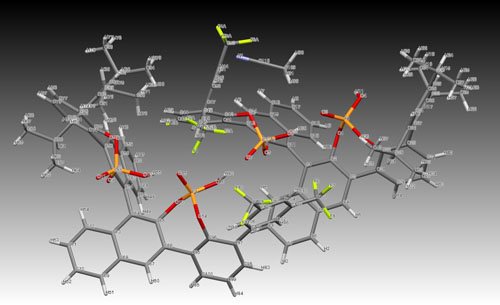 X-ray structure of our recent development
X-ray structure of our recent development
Selected Publications
-
S. Oishi, T. Fujinami, Yu.Masui, T. Suzuki, M.Kato, N.Ohtsuka, N. Momiyama iScience 25, 105220 (2022).
-
C. Jongwohan, Y. Honda, T. Suzuki, T. Fujinami, K. Adachi, N. Momiyama, “Brønsted Acid-Initiated Formal [1,3]-Rearrangement Dictated by β-Substituted Ene-Aldimines” Org. Lett. 21, 4991-4995 (2019).
-
N. Momiyama, H. Tabuse, H. Noda, M. Yamanaka, T. Fujinami, K. Yamanishi, A. Izumiseki, K. Funayama, F. Egawa, S. Okada, H. Adachi, M. Terada, “Molecular Design of a Chiral Brønsted Acid with Two Different Acidic Sites: Regio-, Diastereo-, and Enantioselective Hetero-Diels-Alder Reaction of Azopyridinecarboxylate with Amidodienes Catalyzed by Chiral Carboxylic Acid-Monophosphoric Acid” J. Am. Chem. Soc. 138, 11353-11359 (2016).
-
N. Momiyama, H. Okamoto, J. Kikuchi, T. Korenaga, M. Terada, “Perfluorinated Aryls in the Design of Chiral Brønsted Acid Catalysts: Catalysis of Enantioselective [4+2] Cycloadditions and Ene- Reactions of Imines with Alkenes by Chiral Mono-Phosphoric Acids with Perfluoroaryls” ACS Catal. 6, 1198-1204 (2016).
-
N. Momiyama, K. Funayama, H. Noda, M. Yamanaka, N. Akasaka, S. Ishida, T. Iwamoto, M. Terada, “Hydrogen Bonds-Enabled Design of a C1-Symmetric Chiral Brønsted Acid Catalyst” ACS Catal. 6, 949-956 (2016).
-
N. Momiyama, T. Narumi, M. Terada, “Design of a Brønsted Acid with Two Different Acidic Sites: Synthesis and Application of Aryl Phosphinic Acid-Phosphoric Acid as a Brønsted Acid Catalyst” Chem. Commun. 51, 16976-16979 (2015).
-
N. Momiyama, T. Konno, Y. Furiya, T. Iwamoto, M. Terada, “Design of Chiral Bis-phosphoric Acid Catalyst Derived from (R)-3,3’-Di(2-hydroxy-3-arylphenyl) binaphthol: Catalytic Enantioselective Diels-Alder Reaction of α,β-Unsaturated Aldehydes with Amidodienes” J. Am. Chem. Soc. 133, 19294-19297 (2011).
-
N. Momiyama, M. W. Kanan, D. R. Liu, “Synthesis of Acyclic α,β-Unsaturated Ketones via Pd(II)-Catalyzed Intermolecular Reaction of Alkynamides and Alkenes” J. Am. Chem. Soc. 129, 2230-2231 (2007).


 X-ray structure of our recent development
X-ray structure of our recent development