Research Theme
Exploitation of Novel Spectroscopic Methods for Material and Surface Science
Keywords
Synchrotron Radiation, Operando Spectroscopy, X-ray absorption Spectraoscopy, X-ray Photoelectron Spectroscopy
Modern materials science requires the creation of harmonious materials from various perspectives, including safety and security as well as high performance, and requires detailed evaluation and analysis of the materials for better material design. Recently, a demand for the development of direct operando observation methodology has been desired. We have been developed new measurement methods based on spectroscopic techniques, mainly using X-rays emitted from large-scale accelerators (synchrotron radiation).
We have succeeded in the first measurement of X-ray photoelectron spectra under real atmospheric pressure in 2017. Photoelectron spectroscopy usually requires measurement under high vacuum, but by using hard X-rays with high energy, we were able to develop a photoelectron spectrometer that allows us to measure the spectra under 1 atmospheric pressure. Using this system, we have been studying the deactivation mechanism of fuel cell performance and the cause of poisoning by analyzing the fuel cell under operation, and the surface chemical reaction of CO2 reduction catalysts.
Currently, we are using synchrotron radiation X-rays to study catalytic reactions on solid surfaces and electrochemical reactions at the electrode-electrolyte solid-liquid interfaces by photoelectron spectroscopy and to study low thermal expansion materials by absorption spectroscopy.
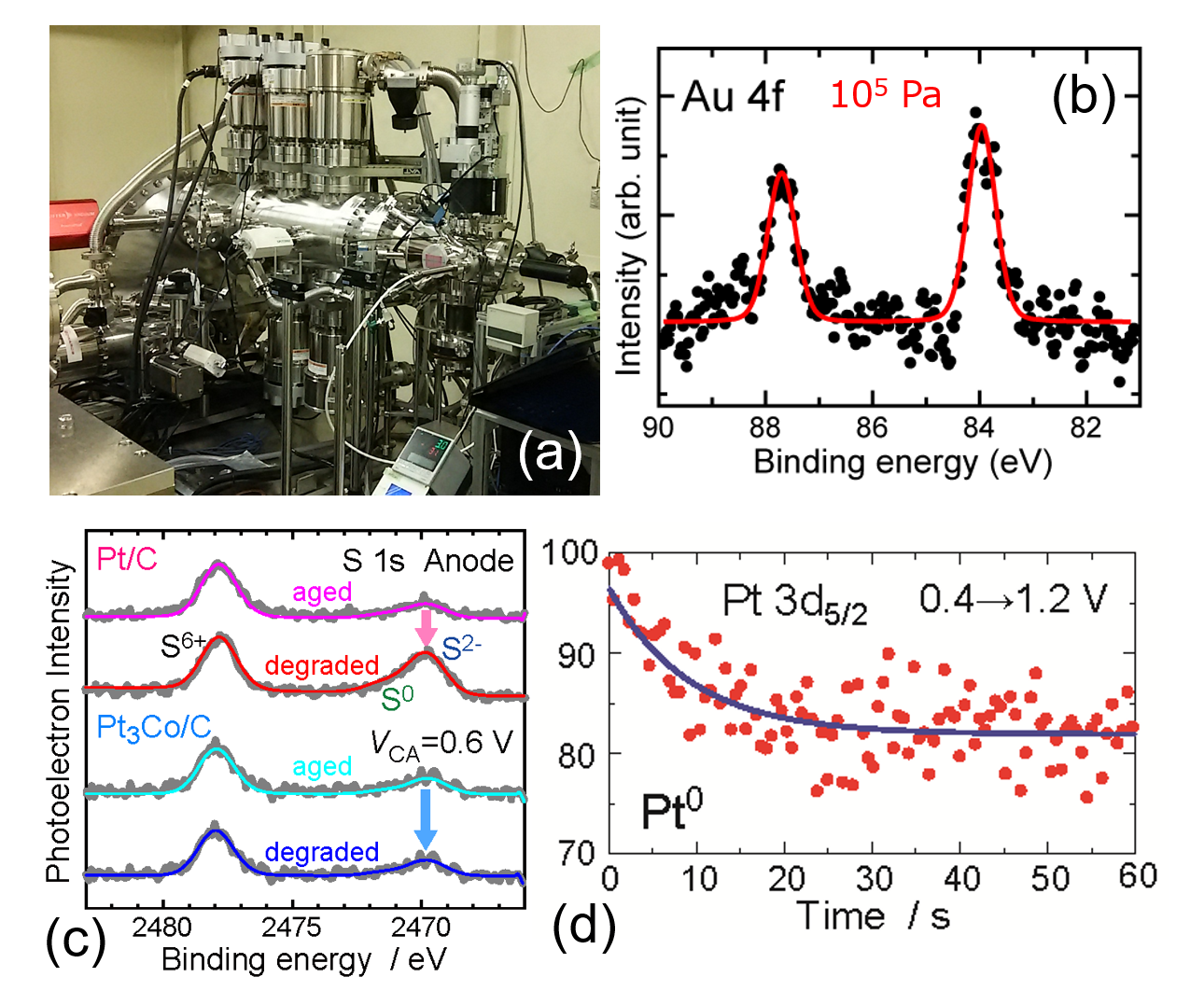
Ambient pressure hard x-ray photoelectron spectroscopic system.
(a) Apparatus installed at SPring-8 Beamline 36XU. (b) Au 4f HAXPES of Au(111) at a real ambient pressure of 105 Pa. (c) S 1s HAXPES of the anode Pt and Pt3Co electrodes of aged (fresh) and degraded polymer electrolyte fuels cells under working conditions. The Pt3Co electrode is found to be more tolerant against S than the Pt electrode. (d) Quick HAXPES measurements of the cathode Pt of the polymer electrolyte fuels cells upon abrupt change of the cathode-anode voltage from 0.4 to 1.2 V. The time resolution is 500 ms.
Selected Publications
-
“Operando Characterization of Copper-Zinc-Alumina Catalyst for Methanol Synthesis from Carbon Dioxide and Hydrogen by Ambient-Pressure Hard X‑ray Photoelectron Spectroscopy” T. Koitaya, K. Yamamoto, T. Uruga and T. Yokoyama, J. Phys. Chem. C 127, 13044 (2023).
-
“Sulfur Poisoning Pt and PtCo Anode and Cathode Catalysts in Polymer Electrolyte Fuel Cells Studied by Operando Near Ambient Pressure Hard X-ray Photoelectron Spectroscopy” S. Chaveanghong, T. Nakamura, Y. Takagi, B. Cagnon, T. Uruga, M. Tada, Y. Iwasawa and T. Yokoyama, Phys. Chem. Chem. Phys. 23, 3866 (2021).
-
“X-ray photoelectron spectroscopy under real ambient pressure conditions” Y. Takagi, T. Nakamura, L. Yu, S. Chaveanghong, O. Sekizawa, T. Sakata, T. Uruga, M. Tada, Y. Iwasawa and T. Yokoyama, Appl. Phys. Exp. 10, 076603 (2017).
-
“Anharmonicity and Quantum Effects in Thermal Expansion of an Invar Alloy” T. Yokoyama and K. Eguchi, Phys. Rev. Lett. 107, 065901 (2011).
-
“Magnetic circular dichroism near the Fermi level” T. Nakagawa and T. Yokoyama, Phys. Rev. Lett. 96, 237402 (2006).