サイト内検索
分子研について
研究情報
共同利用案内
- 共同研究・施設利用案内
- 申請概要
- 共同研究受入研究室・利用装置一覧
- WEB申請
大学院
- 大学院教育
- 大学院案内
- 受験生向け情報
- 他大学の学生の受け入れ
- 在校生向け情報
お知らせ
2023/07/31
プレスリリース
Enantioselection with neither chiral catalysis nor chiral ingredients (absolute enantioselection) has been one of the most active topics of interest but its experimental realizations have been challenging. A team led by researchers at the Institute for Molecular Science (IMS) demonstrated the enantioselectivity of helical supramolecules consisting only of achiral molecules solely by exploiting chiral-induced spin selectivity (CISS) effect. The helicity of the supramolecules is created not by microscopic molecular arrangements but by mesoscopically introduced dislocations. Now CISS effect has been revealed to be relevant for the wider class of chirality ranging from microscopic to mesoscopic length scales.
Matter behaves differently at various scales. At the microscopic, the behavior of molecules and their atomic constituents can shift drastically simply because the scale is so small that any change is major. Even without changing variables, their properties may appear vastly different at the microscopic scale than at the macroscopic. The question is if the reactions that researchers have exploited previously, but only for microscopic targets can be applied for the targets with different scales. This multifunctionality is essential to produce safer chemicals or develop advanced electronics across the various scales.
Now, a team led by researchers at the Institute for Molecular Science (IMS), one of Japan’s National Institutes of Natural Sciences, has found that at least one such technique called the chiral-induced spin selectivity (CISS) effect may have broader applications than just on microscopic reactions.
They published their findings on JULY 27th in Nature Communications.
At the microscopic scale, molecules have chirality — a left or right handedness. While close to mirror images of one another, they cannot be superimposed upon one another. Similar to how a human’s left and right hand are mirror images, they don’t have an exact center of symmetry. In chemical reactions, the produced substances have equal amounts of left-handed and right-handed molecules if there is no chiral catalysis, something to tip the handedness in one side or the other. However, researchers can find way to control the chirality of the produced substances by using chirality-specific reaction without relying on chiral catalysis.
The research team focused on the CISS effect, in which the spin of electrons plays a definitive role in controlling a system’s chirality. Spin, the electron’s intrinsic angular movement, manifests in one of two directions. If the spin of two electrons is different, the electrons can occupy the same space. If it’s the same, the electrons repel each other — like the resistance produced when pressing the same poles of two magnets together. When electrons move through a chiral system, a specific handedness prefers a specific spin because of how the CISS effect influences chiral interactions. In other words, the CISS effect can be used to filter out electrons with one type of spin and the molecules with specific chirality that prefer that spin.
“We found that the CISS effect has been recognized as a new influence, giving enantioselectivity of the chiral molecules without using any chiral catalysis,” said co-corresponding author Takuro Sato, assistant professor at IMS. “However, we have only applied the CISS-based enantioselection to microscopic targets such as chiral molecules, so far, and only to select one of the enantiomers from the mixture. So, whether we can preferentially synthesize a one-sided chiral aggregation at a larger scale only from achiral components through the CISS effect remains an important open question.”
In other words, can researchers use this selectivity to not only sort molecules, but to build them?
To test this, Sato and his team focused on the superstructure of cobalt phthalocyanines, an organometallic molecule frequently used in such electronic devices as light-emitting diodes. Critically, Sato explained, cobalt phthalocyanines are not chiral, meaning they can be superimposed on themselves. The researchers conducted physical vapor deposition technique to allow the molecules to reconstitute as crystallized supramolecules — chemical systems comprising multiple molecules — on silicon substrates covered by magnetic nickel. This process produced helical superstructures with both right- and left-handed chirality, which the researchers confirmed via scanning electron microscopy.
They then placed magnets under the substrates, directing a specific pole to face the substrate to control the directional magnetism of the nickel. Theoretically, if the CISS effect could work in the developed supramolecules, Sato said, the magnetized nickel would interact with the synthesized helical supramolecules. It worked. On the substrates with the south pole of the magnet situated closer, the supramolecules demonstrated preferential left-handedness, while a closer north pole preferentially induced right-handedness. No handedness was preferred in the absence of magnets.
“By exploiting enantiospecific interaction due to CISS, we demonstrate enantioselectivity in the assembly of mesoscale helical supramolecules consisting of achiral cobalt phthalocyanines,” Sato said. “These observations mean that the application of CISS-based enantioselectivity is no longer limited to systems with microscopic chirality but is expanded to the one with mesoscopic chirality.”
Prior experiments from other researchers, Sato noted, generated chiral flow in a classical manner, meaning under the laws of physics that govern matter at the macroscopic scale.
“In contrast, our experiments here utilized the purely electromagnetic field in a quantum manner to prepare the chiral field,” Sato said. “These two examples strongly suggest the universality of the enantioselectivity based on chiral influence that is independent of the detail of the fields.”
Sato said the researchers plan to continue investigating CISS effect applications in the different systems of large-scaled helices and how their findings might apply to the development of advanced optics and sensor technologies.
Other authors include Hiroki Aizawa, Taketoshi Minato and Hiroshi M. Yamamoto, Institute for Molecular Science; Saori Maki-Yonekura, Koji Yonekura and Kiyofumi Takaba, Biostructural Mechanism Laboratory at RIKEN. Co-author Tasuku Hamaguchi was also affiliated with the Biostructural Mechanism Laboratory at the time of research and is now with the Institute of Multidisciplinary Research for Advanced Materials at Tohoku University. Sato, Aizawa and Yamamoto are also affiliated with the Graduate University for Advanced Studies. Yonekura is also affiliated with Tohoku University and the Advanced Electron Microscope Development Unit at the RIKEN-JEOL Collaboration Center.
The Japan Society for the Promotion of Science, the Daiko Foundation, and the Research Support Project for Life Science and Drug Discovery supported this research.
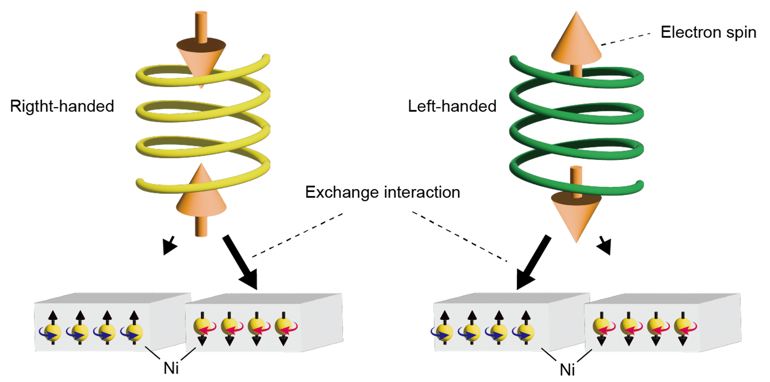
Concept of CISS-based enantioseparation for helical supramolecules by using magnetized substrates.
Emergent spins via CISS effect in chiral objects interact with the magnetized substrates in an enantiospecific manner. The orientation of the anti-parallel spin pair is prescribed by the handedness of the system. The black arrows pointing from the chiral molecules to magnetic substrates indicate the interaction between them. Longer arrows represent stronger interaction.
Authors: Hiroki Aizawa, Takuro Sato, Saori Maki-Yonekura, Koji Yonekura, Kiyofumi Takaba, Tasuku Hamaguchi, Taketoshi Minato, and Hiroshi M. Yamamoto
Journal Name: Nature Communications
Journal Title: “Enantioselectivity of discretized helical supramolecule consisting of achiral cobalt phthalocyanines via chiral-induced spin selectivity effect”
DOI: 10.1038/s41467-023-40133-z
JSPS KAKENHI(19H00891), Daiko Foundation, JST-Mirai Program(JPMJMI20G5), Research Support Project for Life Science and Drug Discovery (Basis for Supporting), Innovative Drug Discovery and Life Science Research (BINDS)) from AMED(JP22ama121006), ARIM(JPMXP1222MS5015)
【On the research】
Institute for Molecular Science
Takuro Sato
takurosato_at_ims.ac.jp
Institute for Molecular Science
Hiroshi M. Yamamoto
yhiroshi_at_ims.ac.jp
(Please replace the "_at_" with @)
【media contact】
Institute for Molecular Science, NINS
press_at_ims.ac.jp
Tohoku University, IMRAM
press.tagen_at_grp.tohoku.ac.jp
(Please replace the "_at_" with @)