| 概 要 |
In the last ten years, many alternatives relying on organic molecules have been explored that could replace traditional solar cells made of inorganic semiconductors. Organic cells are thin, flexible, and cheap-to-produce, but suffer from inherent limitations of organic materials, in particular their low carrier mobility. Nevertheless, the record power conversion efficiency recently reported is as high as 12% [1].
Solar cells using liquid crystal (LC) films of donor-acceptor (DA) molecules are a new approach, in which the ratio of DA interface-to-volume is maximized, leading to fairly good performances [2]. The motivation is to make the distance to the D-A interface shorter than the exciton diffusion length (typically 10 nm), and to use the self-organization of LC's for higher carrier mobility. We have studied LC films of bisthiophene-derivatives forming D and perylenendiimide as A, by femtosecond transient absorption (TA) spectroscopy. Due to the strong electronic coupling between D's the initial laser excitation, selectively tuned in the absorption of D, excites a coherent superposition of many D molecules (exciton) that decays within 60 fs into a charge transfer (CT) state, that localizes on a slower 0.4 ps time scale. However, while the CT formation is ultrafast and efficient, the time-resolved spectroscopy shows that the CT states recombine within less than 50 ps, i.e. on a time scale too short to allow significant carrier transport [3].
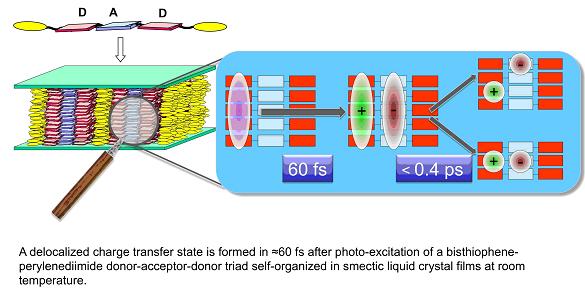
We will present results obtained for a new type of donor family incorporated in the DA's that incorporate moieties with different electron- donating and electron-deficient character, thereby offering a handle to control the localization of HOMO and LUMO orbitals. A nematic liquid crystal state is formed at room temperature. CT lifetimes larger than 2 ns are now observed and the results are rationalized within the Marcus theory for charge transfer.
[1] Heliatek press release, http://www.heliatek.com/newscenter/latest_news/, January 16, 2013
[2] L. Bu and Al., J. Am. Chem. Soc., 2009, 131, 13242-13243
[3] T. Roland and Al., Phys. Chem. Chem. Phys, 2012, 14, 273-279
|